Suggestion of a transition state between propene and cyclopropane
Now we are going to use all our knowledge in pratical terms.
Training with interconversion of propene
Find the transition state between the two forms of propene, and check our results...(as usual)
Let's try with propene <--> cyclopropane
What can we learn in literature ? Two forms for the TS
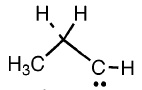
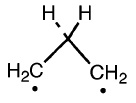
In literature we find two forms for the TS : the propylidene and the trimethylene, that is the singlet and the triplet form, so we have to try to optimize our TS with this two possibilities.
The triplet
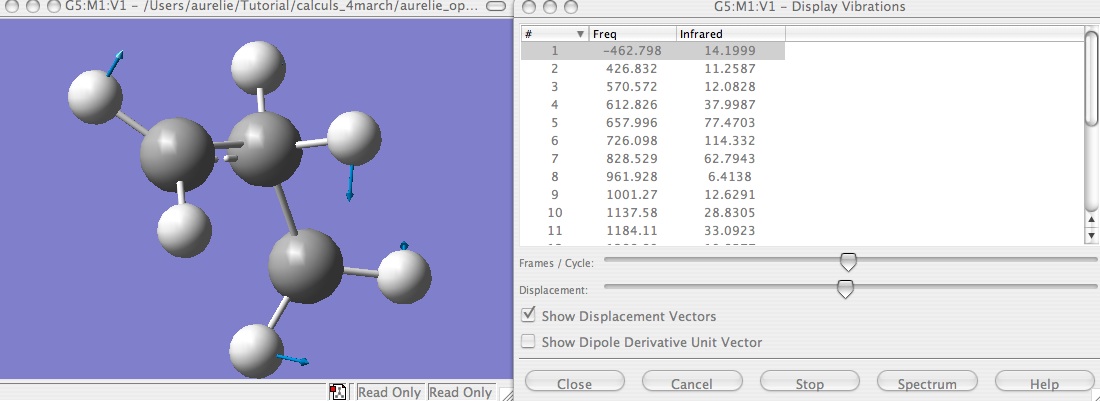
First step : try to optimize with TS Berny.
It does not work in the simpliest way, because the optimized parameters can not be found. So try with Calculate force constant ---> always. This indication ask Gaussian to calculate the gradient in each step and so it is easier to go to the TS, furthermore, because of Gaussian calculate the gradients, you get immediately the frequencies in the file .log. But this calculation is very expensive, so can just use it with this small molecule.
You get just one imaginary frequency, and thanks to the animation of the frequencies you can check that the displacement vectors are in agreement with the theory of the displacement of the H.
Now try to visualize the displacement of the H.
In this aim, select Job type --> IRC, then you can select in which direction you want to look at the reaction in our case select Forward only. Tick the case Compute more points specifying N = 27. For the force constants ask for Read from .CHK (the calculation that you did for the optimization would serve here : Gaussian will take the calculation that it did already, in this goal you just have to specify in Link 0 the name of the previous file .chk). Choose your method, save and run the calculation.
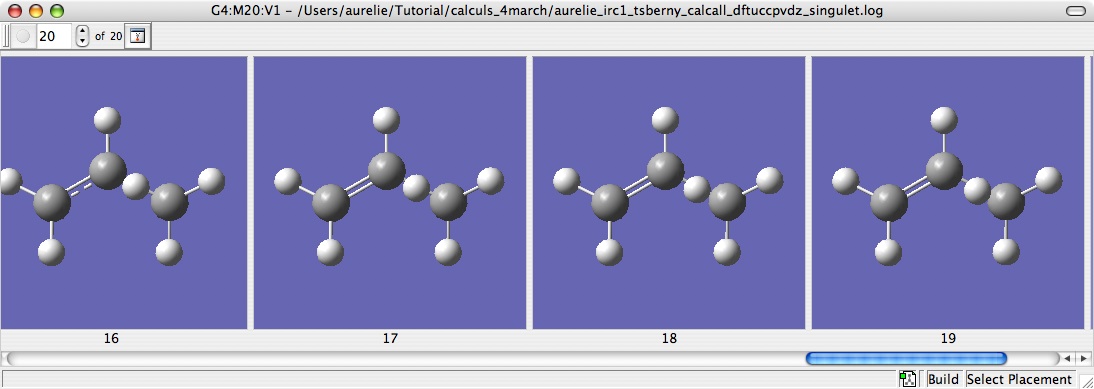
The singlet
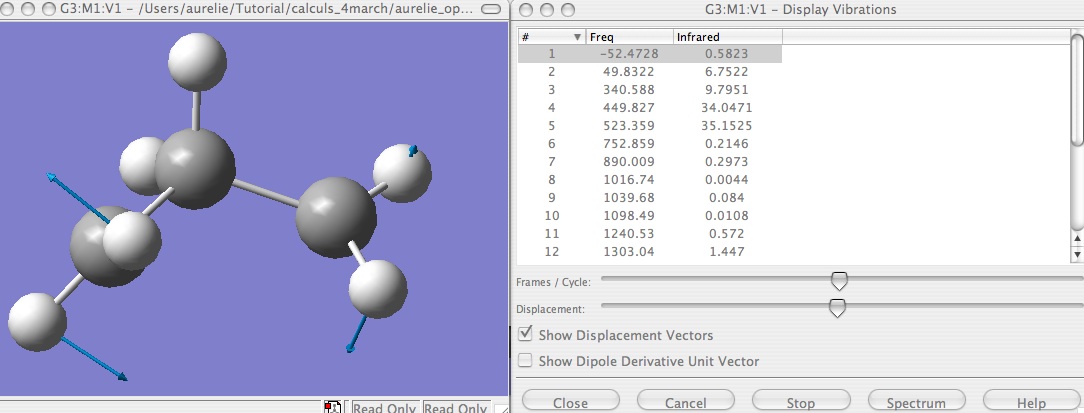
First step : try to optimize with TS Berny.
I obtained one imaginary frequency, so i have the true TS, and he displacement of the atom in this TS seems to good the good one.
If you do not succeed the optimization with TS Berny try the QST2 method, in this aim, have a look at How to use QST2.
If you are very unlucky it could not work again, so test your last chance QST3, have a look at How to use QST3.
Then, run the IRC calculation in the same way as explain previously.
Results
At the end of the calculation open the .log with GaussView, and select IRC in Results. All the 20 steps of the calculation are drawn and you can see the reaction.
And he jump of the H could distinctly be seen.
Compare the energy of our TS with the literature
By running calculations with DFT cc-pvdz basis set (Why this basis set?). I got the results below.
| Energy (Hartree) | ECyclopropane - EPropene (kcal/mol) | ECyclopropane - ETS(kcal/mol) | EPropene - ETS(kcal/mol) | |
| Propene | -117.832569 | 9.59400039 | ||
| Cyclopropane | -117.81728 | 9.59400039 | ||
| TS - singlet | -117.697912 | -74.90461368 | -84.49861407 | |
| TS - triplet | -117.730694 | -54.33358086 | -63.92758125 |
Output fair
Optimization of propene as a minimum : Media:aurelie_opt_min_calcall_dftccpvdz_propene.log
Optimization of cyclopropane as a minimum : Media:aurelie_opt_min_calcall_dftccpvdz_cyclopropane.log
Optimization of the triplet as a transition state : Media:aurelie_opt_tsberny_calcall_dftuccpvdz_triplet.log
Optimization of singulet as a transition state : Media:aurelie_opt_tsberny_calcall_dftuccpvdz_singulet_end1.log
Get back the results found in an article
Some articles concerning the subject
Structural isomerization of cyclopropane: a new mechanism through propylidene
Structural and geometrical isomerizations of cyclopropane. Quantum chemical and RRKM calculations
A theoretical study on the isomerization of cyclopropane to propene with ab initio and DFT methods
Appendix 1 : TS Berny, QST2 or QST3 ??
These three methods are totally independent. Each one is as accurate as the next: they just constitute three different ways to find a TS and so there are three different codes in Gaussian. So you are totally free to choose your favourite method!
There are advantages of having three different methods: if one doesn't work then there exist other ways for optimizing a TS. There are two things you can say to yourself if all three don't work for the first time: "Maybe the TS that I've conjectured doesn't exist ", and, "Maybe the symmetry of my molecule is preventing me from finding a TS, ergo break the symmetry".
How to use QST2
How to use QST3
Back to Resgrp:comp-photo-c3h6-tutorial
Or go to : Some other methods : MPn, CCSD The theoretical Background Use Gaussian website : User's reference online Unix reminder