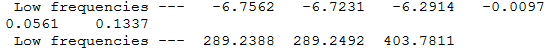RichardZhang
Borane
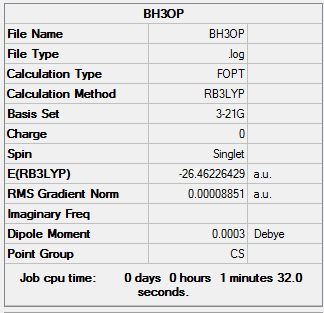
Optimization of Borane(3-21G)
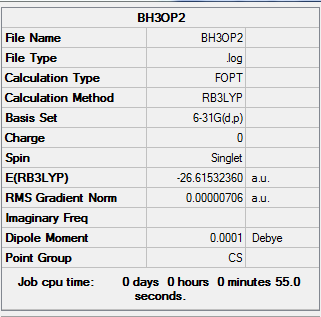
Optimization of Borane(6-31G)
The optimisation file is linked to here
Ng611 (talk) 19:29, 4 June 2018 (BST) You mean 'frequency file' (you've uploaded the frequency file, which is correct, but not labelled it as such).
BH3 |
| Mode | Vibration | Intensity | Symmetry | IR Active | Type |
|---|---|---|---|---|---|
| 1 | 1163 | 93 | A2' | Yes | out-of-plane bend |
| 2 | 1213 | 14 | E' | Yes | bend |
| 3 | 1213 | 14 | E' | Yes | bend |
| 4 | 2582 | 0 | A1' | No | symmetric stretch |
| 5 | 2715 | 126 | E' | Yes | asymmetric stretch |
| 6 | 2715 | 126 | E' | Yes | asymmetric stretch |
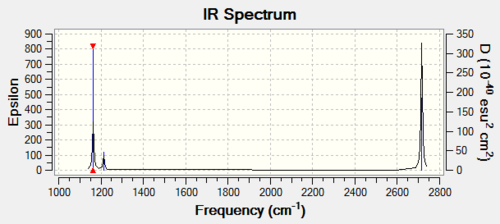
There are less than six peaks in the spectrum even though there are obvious six vibrations. This is because the translation modes are degenerate each other, such as mode 2 and mod 3, mode 5 and mode 6. In addition, the vibration mode 4 is IR inactive, which therefore also missed in the IR spectrum.
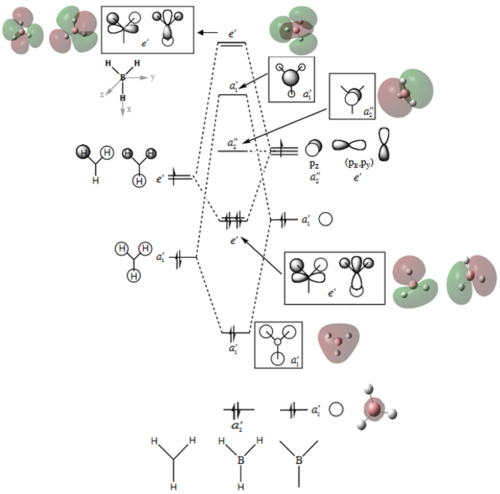
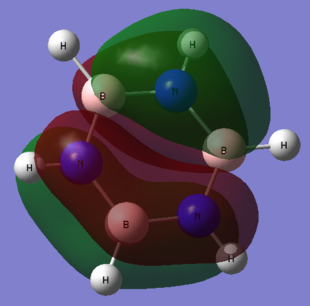
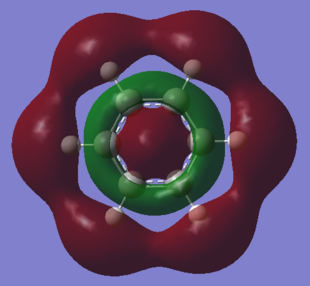
No obvious differences can be observed between the real and LCAO MOs, which means that the qualitative MO theory is quite accurate.
Association energy calculation
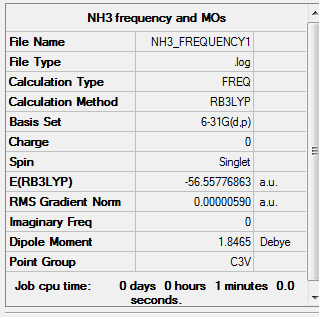
Optimization of Ammonia(6-31G)
1.RB3LYP/6-31G:
The optimisation file is linked to here
Ammonia |
| Mode | Vibration | Intensity | Symmetry | IR Active | Type |
|---|---|---|---|---|---|
| 1 | 1694 | 145 | A | Yes | out-of-plane bend |
| 2 | 1694 | 13 | E | Yes | bend |
| 3 | 1694 | 13 | E | Yes | bend |
| 4 | 3461 | 1 | A1 | No | symmetric stretch |
| 5 | 3589 | 0 | E | No | asymmetric stretch |
| 6 | 3589 | 0 | E | No | asymmetric stretch |
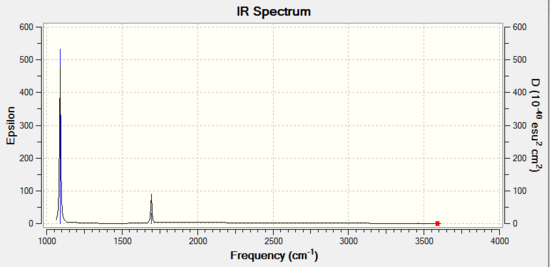
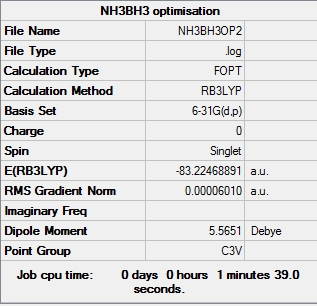
Optimization of Ammonia Borane(6-31G)
1.RB3LYP/6-31G:
The optimisation file is linked to here
Ng611 (talk) 19:32, 4 June 2018 (BST) Remember to upload the correct file! We're looking for frequency log files.
Ammonia Borane |
Calculation process
E(NH3)=-56.55776863 a.u ≈ -1.48492x10^5 kJ/mol
E(BH3)=-26.61532360 a.u ≈ -6.9879x10^4 kJ/mol
E(NH3BH3)=-83.22469012 a.u ≈ -2.18506x10^5 kJ/mol
ΔE = E(NH3BH3)-[E(BH3)+E(NH3)] = -2.18506x10^5+(6.9879x10^4+1.48492x10^5) ≈ -135 kJ/mol
The value of association energy is a negative value and much smaller than the carbon carbon single bond(around 300 kJ/mol) and carbon-carbon double bond(around 600 kJ/mol). Therefore, the B-N dative bond is relatively weak.
Ng611 (talk) 19:31, 4 June 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper).
Borone tribromide
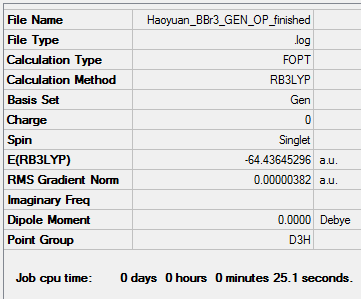
Optimization of borone tribromide(GEN)
1. RB3LYP/GEN:
The optimisation file is linked to here
Boron tribromide |
| Mode | Vibration | Intensity | Symmetry | IR Active | Type |
|---|---|---|---|---|---|
| 1 | 156 | 145 | E' | NO | asymmetric stretch |
| 2 | 156 | 13 | E' | NO | asymmetric stretch |
| 3 | 268 | 13 | A1' | NO | symmetric stretch |
| 4 | 378 | 1 | A2 | Yes | out-of-plane bend |
| 5 | 763 | 0 | E' | Yes | bend |
| 6 | 763 | 0 | E' | Yes | bend |
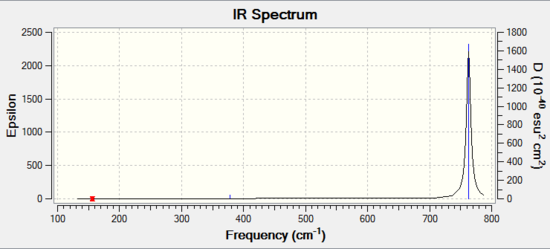
The pps-optimisation of BBr3: DOI:10042/202437
The pps-frequency of BBr3: DOI:10042/202439
Project of aromaticity
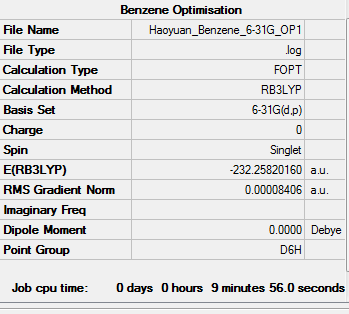
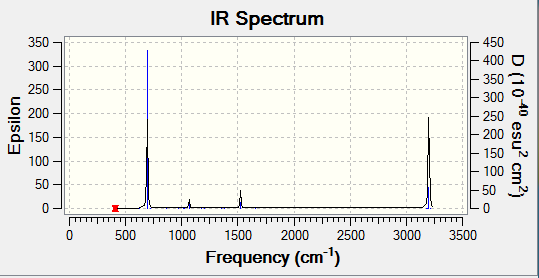
Optimization of Benzene
1.RB3YLP/6-31G:
The optimisation file is linked to here
Ng611 (talk) 19:34, 4 June 2018 (BST) Again, remember to upload frequency lof files.
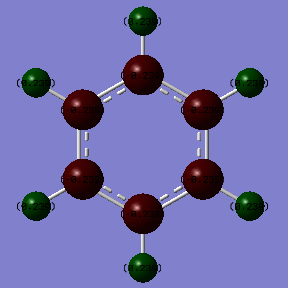
Benzene |
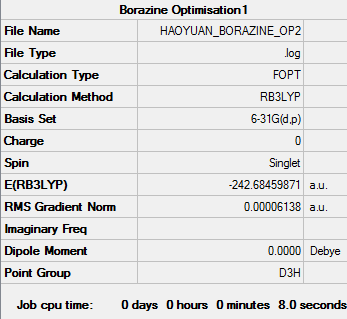
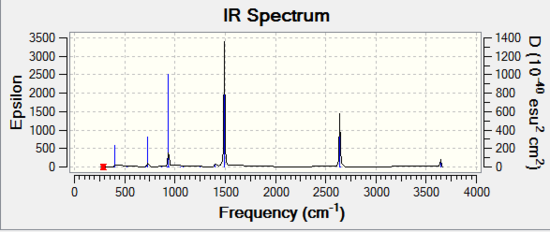
Optimization of Borazine
1.RB3YLP/6-31G:
The optimisation file is linked to here
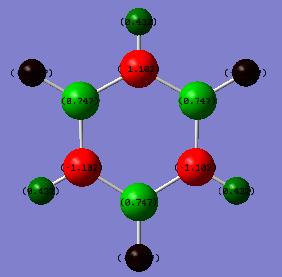
Borazine |
The Natural Bond Orbital analysis
| Atom | Charge/a.u | Electronegativity |
|---|---|---|
| c | -0.239 | 2.5 |
| H | 0.239 | 2.2 |
| Atom | Charge/a.u | Electronegativity |
|---|---|---|
| B | 0.747 | 2.0 |
| N | -1.102 | 3.04 |
| H-N | 0.432 | 2.2 |
| H-B | -0.077 | 2.2 |
According to the diagrams and tables shown above, it is obvious that the charge density in the benzene is evenly distributed. In addition, the charge of the hydrogen is 0.239 a.u and the charge of the carbon is -0.239, which is a big difference. This is mainly because of the electronegativity difference between H(2.2) and C(2.5). As carbon is more electronegative than hydrogen, it will attracts the charges toward itself, which therefore becomes more negative in charge density than hydrogen. For borazine, the distribution of charge density is more complex. The nitrogen is a strong electron withdrawing group as the electronegativity of N is around 3.04 which is much larger than the electronegativity of hydrogen(2.2)and boron(2.0). Therefore, it will pulls the charge density towards itself and away from the boron and hydrogen.
Ng611 (talk) 19:35, 4 June 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry?
Ng611 (talk) 19:41, 4 June 2018 (BST) Well done for comparing the correct MOs by shape and not energtic ordering (which is not necessarily reliable). I would include a brief discussion of the overall symmetry of the molecule to improve this section further. Perhaps also consider dicussing the constituent AOs that form the MOs and the overall symmetry of the MO. I'm also not sure your discussion of MO 24 is particularly useful as it's quite high in energy.
The aromaticity is applied in the organic chemistry to descirbe a cyclic, flat molecule with a ring of resonance bonds. These bonds induces more stability other types of structure or arrangements of atoms. In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often made of alternating single and double bonds in a ring. This configuration allows for the electrons in the molecule's pi system to be delocalized around the ring, increasing the molecule's stability.
Ng611 (talk) 19:41, 4 June 2018 (BST) Not nearly enough discussion here. You should include some thoughts on how the modern conceptual model of aromaticity differs from Huckel's model to improve the section.
Ng611 (talk) 19:41, 4 June 2018 (BST) An adequate report. Your discussion on aromaticity was missing a great deal of detail, and you missed out on easy marks by uploading the wrong log files. However, your calculations were accurately and correctly performed -- some additional discussion and analysis would have made this into a truly excellent piece of work.