It:Melatonin
Properties of Melatonin1
| Properites | ||
| IUPAC Systematic Name | N-[2-(5-methoxy-1H-indol-3-yl)ethyl]ethanamide | |
| Chemical Name | 5-methoxy-N-acetaltryptamine | |
| Synonyms | Circadin, MT6, Mela-T, Melatol, Melatonex, Melatonine, Melovine, Night Rest, Regulin | |
| Molecular formula | C13H16N202 | |
| Molecular Mass | 232.278 gmol-1 | |
| Melting Point | 117oC | |
| Type of Substance | Neurohormone | |
| CAS Registry Number | 73-31-4 | |
| SMILES string | CC(=O)NCCC1=CNC2=C1C=C(C=C2)OC | |
| Colour | Clear Solid | |
Melatonin, (C13H16N202), or 5-methoxy-N-acetaltryptamine is considered to be the ultimate route towards an uninterrupted sleep. It also controls other vital bodily functions, including the metabolism rate and ones sex drive. Perhaps most excitingly however, melatonin is thought to hold the key to the door behind which the prospect of human life extension lies. The idea of being able to extend the human lifespan makes melatonin one of the most widely researched topics today.
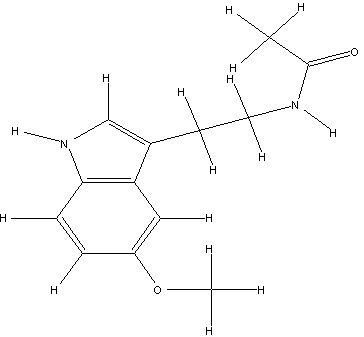
The 3D structure,(figure 1) and chemical structure, (figure 2) of melatonin is shown below, alongside its physical and chemical data, (above). This structure was determined via X-ray diffraction. A single melatonin crystal can be isolated after crystallising it in a benzene solution. Infrared and 1H NMR spectroscopy can be used to analyse the compound. Both techniques show evidence of hydrogen bonding between nitrogen and hydrogen. This provides an adequate reason for the higher than expected boiling point of melatonin, (1160C – 1180C).
Melatonin is the hormone that helps to regulate sleep-wake cycles, and can be found within all living creatures. Besides this key role, melatonin is thought to have many other useful properties, and minimal side effects. As a result, it is available as an over the counter drug, even though it is secreted naturally within humans. While the natural form can be contaminated by viruses, the synthetic product is often used to eliminate sleeping disorders and to minimise the effects of jet lag. Older people may find it particularly useful because as humans age, the amount of melatonin secreted in the body decreases. This gives some explanation as to why young people find it easier to sleep than older people.
For humans specifically, the neurohormone is naturally synthesised by the enzyme 5-hydroxyindole-O-methyltransferase from tryptophan, an amino acid essential in human nutrition.
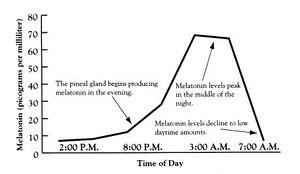
The secretion occurs by the pineal gland, (figure 3), in the brain, the gastrointestinal tract and the retina as the fall of darkness is registered by the eyes. The two conditions required for the pea-sized pineal gland to produce the hormone. Firstly, it must be dark, and secondly the time must be right, (i.e. between approximately 09.00 and 21.00). The latter condition is illustrated in the diagram below, (figure 4).
The levels of melatonin in the circulatory system are easily monitored as the hormone is non-species specific.
This allows a direct comparison to be made between pineal activity in species with varying breeding patterns and the amount of melatonin being produced. Many techniques can be used to analyse the levels of melatonin in the blood, of which bioassay, fluorometry, mass spectroscopy and gas chromatography are the most prominent. Of these, gas chromatography and mass spectroscopy are probably the most useful as they are highly specific and highly sensitive, giving an error of just ±0.01pg.
Melatonin can be used in a wide variety of disciplines, including:
2. Visual impairment treatment5
3. Neurological disorder treatment6
5. Dietary supplements
6. Cancer treatment
7. Strengthening the immune system
8. Human life extension
Although melatonin is one of the least toxic substances known to man, the drug has not been approved by the Food and Drug Administration. As a result, there has been no indication by a regulating body as to what a safe dosage of melatonin is. Indeed, in many cases, the amount of the drug taken to induce an effect varies greatly from person to person. An experimental safe dosage is said to be 1.5mg, and should be taken before sleeping. However, doctors have administered dosages from 1mg to 200mg, and there is no evidence to suggest that over dosing leads to increased side effects. It is clear however, that even at extremely high dosages, (300mg), the negative side effects have been minimal. These include simply drowsiness and slower reaction times. Muscle, mood and sex life enhancement, along side energy boosting properties are amongst the positive side effects of melatonin.
Melatonin is still in the early stages of a highly rigorous analytical process, designed to assess its safety as a drug for humans. Regardless of this seemingly harmless substance, scientists have advised pregnant women, those with severe allergies, mental illnesses and immune system cancers not to take it.
==3D Structure== figure 1
==Chemical Structure== figure 2
References
1. http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00742.txt
3. http://www.ch.ic.ac.uk/local/projects/s_thipayang/+start.html
4. http://ep.bmj.com/cgi/content/full/90/3/ep74
5. http://ep.bmj.com/cgi/content/full/90/3/ep74
6. http://ep.bmj.com/cgi/content/full/90/3/ep74
7. http://ep.bmj.com/cgi/content/full/90/3/ep74
General Information
1. Lewy AJ, Lefler BJ, Emens JS, et al., CHRONOBIOLOGY INTERNATIONAL 23 (1-2): 403-412 2006
2. http://en.wikipedia.org/wiki/Melatonin
3. http://www.ch.ic.ac.uk/local/projects/s_thipayang/+start.html
4. Rufo-Campos M: REVISTA DE NEUROLOGIA 35, S51-S58 Suppl. 1 SEP 2002