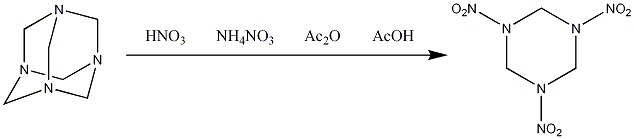It07:RDX
| It07:RDX | |
|---|---|

| |
| General | |
| Systematic name | 1,3,5-trinitro-1,3,5-triazacyclohexane |
| Other names | 1,3,5-trinitroperhydro-1,3,5-triazine
Cyclonite, Hexogen, RDX |
| Molecular formula | C3H6N6O6 |
| SMILES | C1N(CN(CN1[N+](=O)[O-])[N+](=O)[O-])[N+](=O)[O-] |
| Molar mass | 222.117 g/mol |
| Appearance | colourless crystalline solid |
| CAS number | 121-82-4 |
| Properties | |
| Density | 1.82 g/cm³ |
| Solubility in water | 0.0426 g/L |
| Melting point | 205.5°C |
| Ignites at: | 234°C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
3D Structure
Overview

The nitroamine RDX, also known as cyclotrimethylenetrinitramine, is an explosive compound commonly used in industry and the military. It is safe to store without the requirement of special conditions and will not detonate without the use of a detonator. In its pure form it is a white crystalline solid although it is rarely used pure, but rather as a mixture with other compounds. The most common mixture, composition-4 (C4), has 1.34 times the explosive power of trinitrotoluene (TNT)[1].
Name
There are several interpretations of the name RDX, a common one being Royal Demolition eXplosive. Another possibility is Research Department composition X. The argument here is that most military explosives made in the UK were given a number after the letters RD for Research Department. It is unknown why a number wasn't assigned to RDX, but one theory is that the naming department blew itself up with it[2].
History
The first documented usage of RDX was in 1890 when Hans Henning offered it to a patient as a medicine. It was not until 1920 that the explosive properties of the compound were discovered[2]. It saw wide-scale use by both sides during World War II, primarily in mixtures with TNT, which is known as composition B. It continued to be used in military operations throughout the years after World War II, usually in C4.
Composition of C4
The components of C4[3] are present in the following composition:
RDX - 91%
Di(2-ethylhexyl) sebacate - 5.3%
Polyisobutylene - 2.1%
Motor oil - 1.6%
The Di(2-ethylhexyl) sebacate is a plasticiser and the polyisobutane is the binder. When mixed with RDX, these make the compound highly malleable and allow it to be moulded into many different shapes which also has the effect of controlling the direction of the explosion. In addition, the plasticiser and binder make the explosive more stable to shock and heat so that it's safe to carry around without the danger of it detonating. In fact, it will not detonate even when shot or brought near a fire. If set on fire, it will just burn slowly, which led to soldiers using it as an improvised cooking fire during the Vietnam war. RDX, and also C4, can be made to explode by applying heat and pressure at the same time.
Synthesis
The most common synthesis[4] of RDX is known as the Bachmann process and was developed in the USA.
This reaction also gives a 8-12% HMX as an impurity[5], as shown by its high performance liquid chromatogram (HPLC) below. The conditions of this reaction can be changed to give a higher yield of HMX compared to RDX, which is discussed on the HMX page.
There are other possible syntheses for RDX, such as the direct nitration of hexamine using only nitric acid and without the use of acetic anhydride, but this is a lot more dangerous and gives lower yields of RDX[6]. With the method given above, there is no danger of the product spontaneously exploding during the synthesis. More details on alternative methods for RDX synthesis can be found here:
http://yarchive.net/explosives/rdx_make.html
Usage

Today, RDX is still used in the military as C4 charges. This is possible due to the nature of the compound's decomposition, which very rapidly produces nitrogen and carbon dioxide gases. During their initial production, these gases expand at 8,050 meters per second, or 26,400 feet per second[3], destroying everything in the surrounding area by applying huge amounts of force.
Civilians use the compound as the explosive in fireworks and demolition charges for demolishing disused buildings. Additionally, RDX has limited use as rat poison as there is evidence that it causes seizures in both humans and animals if inhaled or ingested in large quantities[5].
High Performance Liquid Chromatography (HPLC)
Here is the HPLC chromatogram[7] for the product of the Bachmann process.
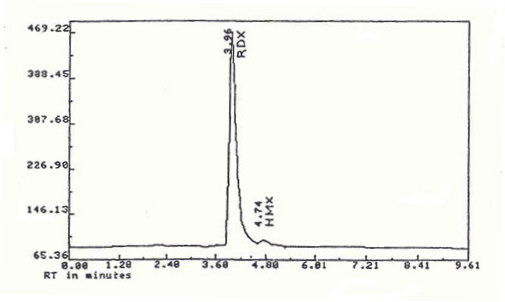
It clearly indicates the presence of the HMX impurity at a slightly higher retention time than RDX and also that the degree of impurity is acceptably small.
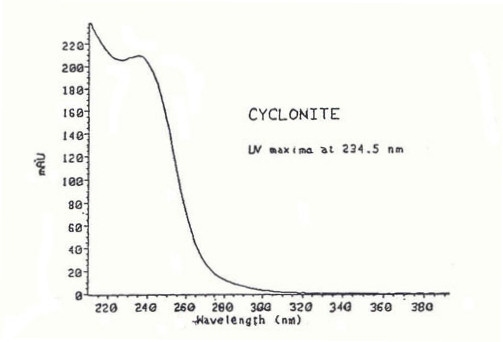
UV spectrum
Here is the recorded UV spectrum[7] for RDX. As it is a colourless solid, it absorbs very weakly in the visible region, so the intensity of the spectrum effectively drops to 0 absorption units after about 320 nm.
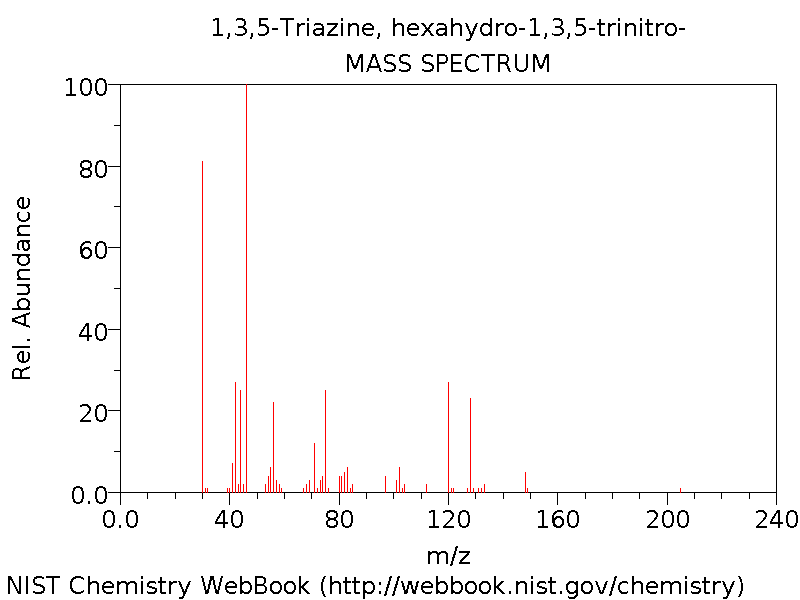
Mass spectrum
Below is the observed mass spectrum[8] for RDX.
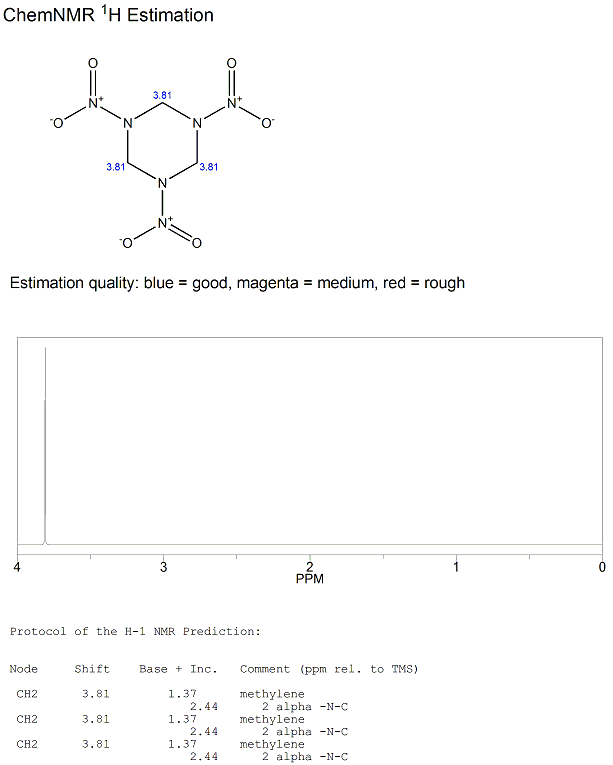
1H-NMR spectrum
RDX gives a very simple 1H-NMR spectrum with only one singlet. There are 3 methylenes, each with 2 hydrogens which are all both chemically and magnetically equivalent to each other so they do not couple with the other methylene hydrogens. Hence, we only observe one singlet.
The predicted 1H-NMR spectrum using prediction software is shown below.
The predicted chemical shift of 3.81ppm for the singlet is in good agreement with the experimentally observed chemical shift[9] of 3.88ppm when recorded in DMSO solvent.
References
- ↑ http://en.wikipedia.org/wiki/C4_explosive
- ↑ 2.0 2.1 http://en.wikipedia.org/wiki/RDX
- ↑ 3.0 3.1 http://science.howstuffworks.com/c-42.htm
- ↑ Russian Journal of Organic Chemistry (Translation of Zhurnal Organicheskoi Khimii), 37(7), 1030-1033; 2001
- ↑ 5.0 5.1 http://www.globalsecurity.org/military/systems/munitions/explosives-nitramines.htm
- ↑ http://yarchive.net/explosives/rdx_make.html
- ↑ 7.0 7.1 http://www.osha.gov/dts/sltc/methods/partial/pv2135/pv2135.html
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=121-82-4
- ↑ J. Chem. SOC. (B), 1968; 6-8