It07:Trinitrotoluene
2,4,6-Trinitrotoluene (TNT)
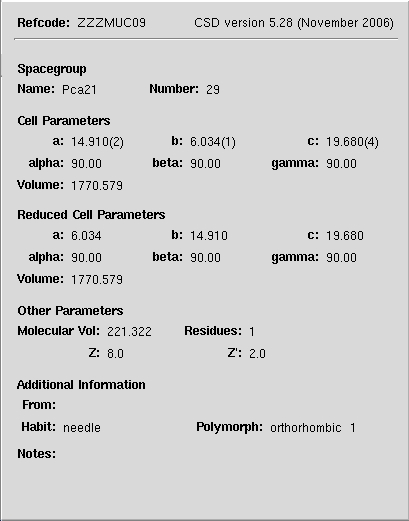
3D structure and crystal unit cell
1
Interactive 3D representation of TNT.
Right click on molecule to explore
| It07:Trinitrotoluene | |
|---|---|
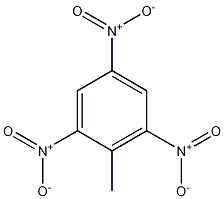
| |
| General | |
| Systematic name | 2-Methyl-1,3,5-trinitrobenzene |
| Other names | 2,4,6-Trinitrotoluene Trotyl; 2,4,6-Trinitromethylbenzene |
| Molecular formula | C6H2(NO2)3CH3 Point group = C2h |
| SMILES | Cc1c(cc(cc1N(=O)=O)N(=O)=O)N(=O)=O |
| Molar mass | 227.131 g/mol |
| Appearance | Yellow(Needle Shaped) |
| CAS number | 118-96-7 |
| Properties | |
| Density | 1.654 g/cm³ |
| Solubility in water | 130 mg/L of H2O (20 °C) |
| Solubility in other solvent | ether;acetone;benzene;pyridine |
| Melting point | 353.35K |
| Boiling point | 568K |
| Acidity (pKa) | {{{pKa}}} |
| Basicity (pKb) | {{{pKb}}} |
| Chiral rotation [α]D | {{{Rotation}}}° |
| Viscosity | {{{Viscosity}}} cP at 25°C |
| Structure | |
| Molecular shape | {{{Mol_Shape}}} |
| Coordination geometry |
{{{Coordination}}} |
| Crystal structure | {{{Crystal_Structure}}} |
| Dipole moment | 0.98 D at 25oC tetrachloromethane solvent |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | {{{Hazards}}} |
| NFPA 704 | {{{NFPA}}} |
| Flash point | {{{Fp}}}°C |
| R/S statement | R: R2 R23 R24 R25 R33 S:S35 S44: ? |
| RTECS number | {{{RTECS}}} |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Related compounds | |
| Other anions | {{{Other_anion}}} |
| Other cations | {{{Ohter_cation}}} |
| Related compounds | {{{Relative_Compounds}}} |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
3D crystal unit cell representation of TNT.
Right click on molecule to explore.
Unit cell paramters in Å
About 2,4,6-Trinitrotoluene (TNT)
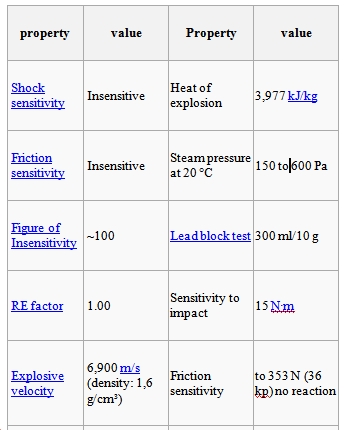
TNT was originally used as a yellow dye by the german chemist Joseph Wilbrand in 1863. Nowadays, the main use for TNT is in explosives. In chemical synthesis it is also used to make charge transfer salts. At the time of its discovery the explosive properties of TNT were not used because it was difficult to detonate and was significantly weaker than other explosives available at the time. TNT is a relatively stable explosive and as a result it can be poured in shell cases when it’s in its liquid phase given that the melting point of TNT is below the one which makes it detonate spontaneously. TNT’s ability to not be affected by water (doesn’t dissolve in water or absorb water) means that it can be effectively utilised underwater. TNT is used as a booster or as a bursting charge for high-explosive shells and bombs 2 .Although pure tnt can be purchased commercially, it is more commonly purchased as a mixture with other compounds such as octol and torpex. TNT decomposes at 295oC to produce nitrogen, carbon monoxide, water and carbon. The reaction has a high activation energy but is very exothermic. The high activation energy is due to the stabilising delocalisation effect involved throughout the whole molecule.
- Enthalpy of fusion = 23948.47 jmol-1 (5)
- Enthalpy of vapourisation = 71175.57 jmol-1 (6)
- Enthalpy of sublimation= 102283.45 jmol-1 (7)
A TNT explosion. Video clip showing an exploding caravan: http://www.youtube.com/watch?v=625OsObNXRc
3
Spectra
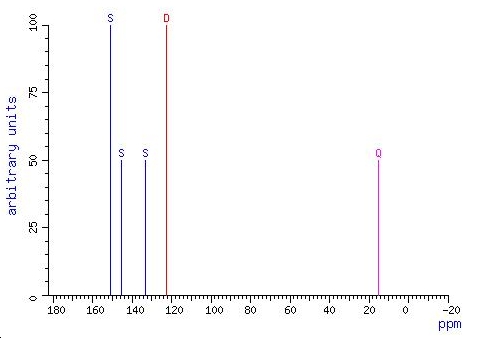
13C NMR spectrum (dimethyl sulfixode solvent at 27oC) 8
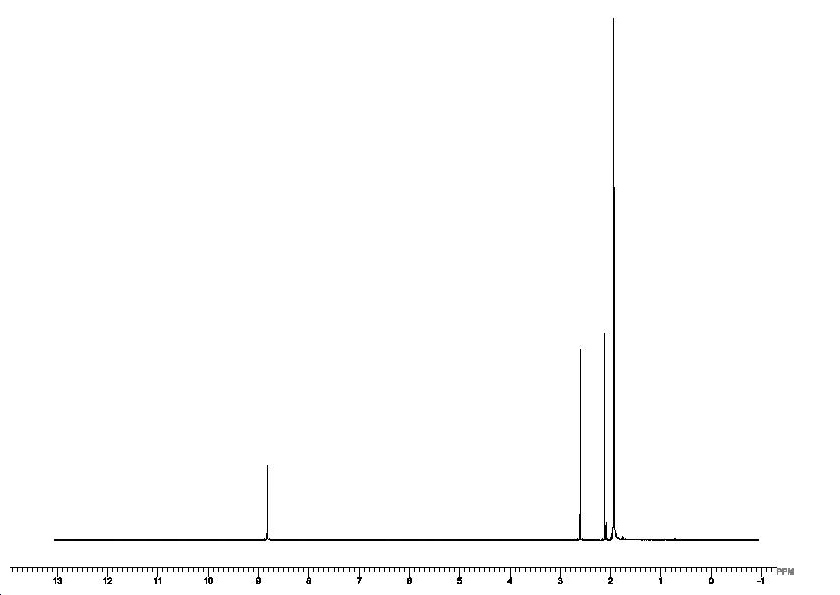
Proton NMR ( acetonitrile solvent at 25oC)10
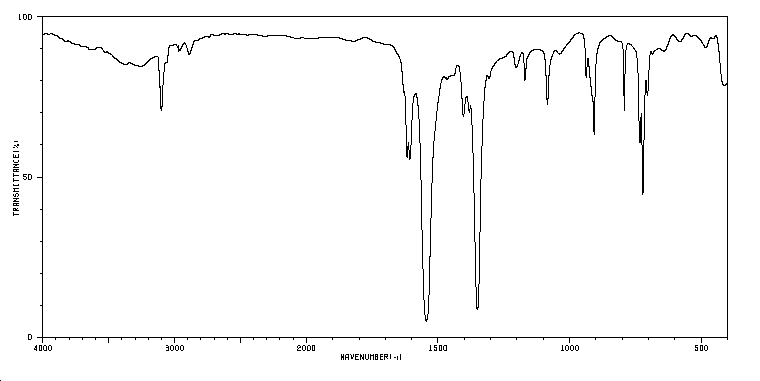
IR spectrum 9
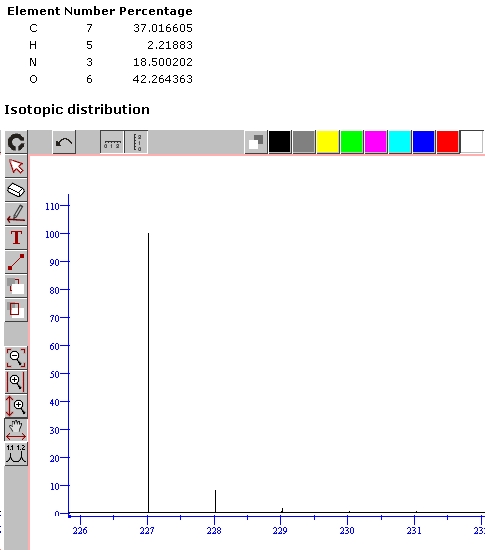
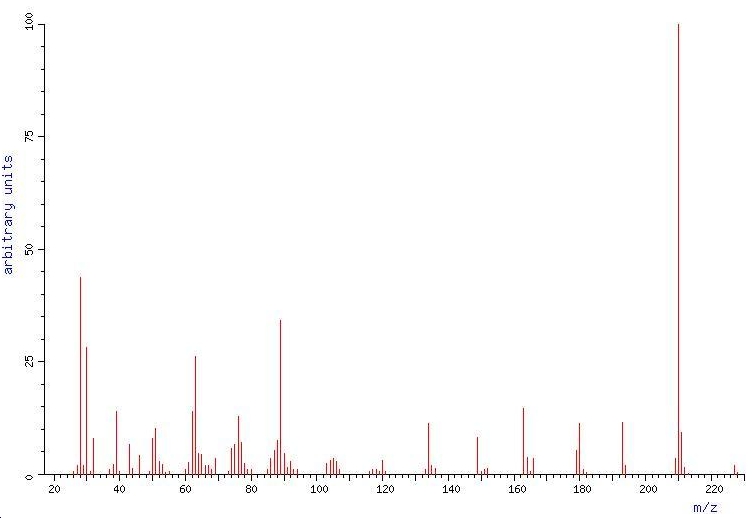
Mass spec 11
Synthesis
General overview
TNT can be synthesised by refluxing toluene with aqua regia (a mixture of concentrated sulphuric acid and concentrated nitric acid) to make dinitrotoluene. Further nitration with nitric acid and 15% oleum leads to successful synthesis of TNT. Using this method, with toluene as the reactant, produces a typical yield of approximately 26% TNT.
Detailed synthesis method 12
Cool 75ml of sulphuric acid (15% oleum) to 273K. Add dropwise 27ml concentrated nitric acid. Warm the acid mixture to room temperature. Add 35.4g of 2,4-dinitrotoluene while gently stirring. Slowly raise the temperature to 363K in a 60 min period. Maintain this temperature for 2 hours. Cool the reaction overnight at 298K. Extract the mixture with 800ml methylene chloride. Neutralise the chloride layer with 500ml with saturated sodium carbonate solution and wash twice with 500ml water. Remove the chloride layer using a rotatory evaporator and recrystallise the solid tnt with ethanol and tetrachloromethane. A yield of 62% can be obtained using this method.
Hazards
4
- carcinogen
- cardiovascular or blood toxicant
- neurotoxicant
- respiratory toxicant
- skin or sense organ toxicant
- Toxic to aquatic organisms
- Harmful to aquatic organisms
- May cause long-term adverse effects in the aquatic environment
- Avoid release to the environment
References
- 1)DOI reference
- 2)http://www.ordnance.org/tnt.htm
- 3)http://www.chemcalc.org/
- 4)http://www.scorecard.org/chemical-profiles/summary.tcl?edf_substance_id=118-96-7#hazards
- 5)Nitta et al, Chem abstr, vol 71, 1951, pg 6448
- 6)Beljajew, Chem abstr, vol 22, 1948, pg 5227
- 7)Lenchitz Velicky, J chem eng data, vol 15, 1970, pg401-403
- 8)ycka, Antonin; Machacek, Vladimir; Jirman, Josef. Res. Inst. Org. Synth., Pardubice-Rybitvi, Czech. Collection of Czechoslovak Chemical Communications (1987), 52(12), 2946-52. CODEN: CCCCAK ISSN: 0366-547X. Journal written in English. CAN 109:148794 AN 1988:548794 CAPLUS
- 9)eelenbinder, John A.; Brown, Chris W. Department of Chemistry and Partnership for Sensors and Surface Technology, University of Rhode Island, Kingston, RI, USA. Applied Spectroscopy (2002), 56(3), 295-299. Publisher: Society for Applied Spectroscopy, CODEN: APSPA4 ISSN: 0003-7028. Journal written in English. CAN 137:33057 AN 137:33057 CAPLUS
- 10)Soojhawon, I.; Lokhande, P. D.; Kodam, K. M.; Gawai, K. R. Department of Chemistry, University of Pune, Pune, India. Enzyme and Microbial Technology (2005), 37(5), 527-533. Publisher: Elsevier B.V., CODEN: EMTED2 ISSN: 0141-0229. Journal written in English. CAN 143:433829 AN 143:433829 CAPLUS
- 11) Sharma, S. P.; Lahiri, S. C. Central Forensic Science Laboratory, Government of India, Kolkata, India. Journal of Energetic Materials (2005), 23(4), 239-264. Publisher: Taylor & Francis, Inc., CODEN: JOEMDK ISSN: 0737-0652. Journal written in English. CAN 143:340765 AN 143:340765 CAPLUS
- 12)DOI reference