It07:HMX
| It07:HMX | |
|---|---|
| |
| General | |
| Systematic name | 1,3,5,7-tetranitro-1,3,5,7-tetrazocane |
| Other names | Cyclotetramethylene-tetranitramine
Octogen, HMX |
| Molecular formula | C4H8N8O8 |
| SMILES | C1N(CN(CN(CN1[N+](=O)[O-])[N+]
(=O)[O-])[N+](=O)[O-])[N+](=O)[O-] |
| Molar mass | 296.20 g/mol |
| Appearance | colourless crystalline solid |
| CAS number | 2691-41-0 |
| Properties | |
| Density & phase | 1.91 g/cm³ |
| Solubility in water | 0.0033 g/L |
| Melting point | 277-278°C |
| Decomposes at: | 280°C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
3D Structure
Overview
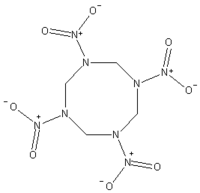
HMX, a white crystalline solid, is an explosive nitroamine which structurally, is very similar to RDX, with the main difference being it is an 8 membered ring with 4 nitrogen atoms and 4 nitro groups. The additional bonds gives it more explosive power than RDX and is in fact the highest energy explosive under large scale production[1]. The main disadvantage is it requires slightly more specific reaction conditions to produce and hence more expensive than TNT and RDX based explosives, but it is useful when maximum destructive power is required.

Name
The name HMX has more than one interpretation, including High-velocity Military eXplosive, Her Majesty's eXplosive and commonly High Melting eXplosive. It is believed that the correct name is simply High Molecular weight rdX[2] seeing as the structure is staggeringly similar to RDX and is essentially a slightly larger version of the same structure.
History
HMX was an accidental discovery by W.E. Bachmann in 1940 as a byproduct in the Bachmann process which was primarily the synthesis of RDX. Bachmann realised that this byproduct was also a high explosive and it took him 3 years to determine the actual molecular structure. Once the structure was identified as what is now known as HMX, Bachmann designed a modified version of his existing synthesis of RDX, which allowed it to undergo mass production and be put to use in military explosives[3].
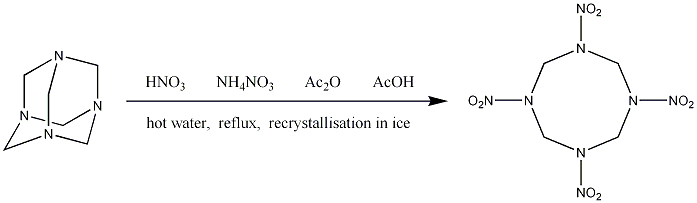
Synthesis
Since HMX is produced in a modified version of the Bachmann process, the same reagents are used as for the synthesis[4] of RDX. Only the conditions are changed slightly.
The ratios of reagents are slightly different to fine tune the yield, but the main difference uses the fact that HMX is highly insoluble in water compared to RDX. After the reagents are mixed, hot water is added and the mixture is refluxed, before finally recrystallising by cooling to 20OC with ice[5]. The result is a 55-60% yield of HMX with RDX as a small impurity[1]. Detailed instructions can be found here:
http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2003/hmx/Structure.htm
Usage
The main use of HMX is in the military as a maximum performance explosive. It is sometimes used pure and produces a shockwave that travels at 9,100 metres per second[6], although usually mixed with another explosive e.g. TNT. The idea here is that the secondary compound has a lower explosive velocity, so the HMX shockwave hits first and inflicts damage, and the shockwave of the secondary compound hits next and finishes off the target. The most common HMX mixture is Octol (See below).
In addition to explosive charges, HMX is used as a propellant in rockets and missiles, detonators for other explosives, det cord (detonator fuse), a fissioning material in nuclear warheads and in explosive nuts and bolts which allow bombs to be released from the wings of an aeroplane[7].
HMX has very limited civilian usage. It is sometimes used in building demolition, but from a civilian point of view, there are cheaper explosives that do the same job just as well.
Octol

Octol is a high explosive mixture[8] of either:
70% HMX and 30% TNT
or
75% HMX and 25% TNT.
HMX has an explosive velocity of 9,100 metres per second, while TNT explodes at 6,900 metres per second (over 2000 m/s difference), so Octol produces two distinct shockwaves in the manner described above, which increases the destructive power.
Another HMX mixture that is sometimes used is Okfol, which is a mixture of 95% HMX and 5% wax, but this slightly reduces the shockwave's velocity to 8670 metres per second[9].
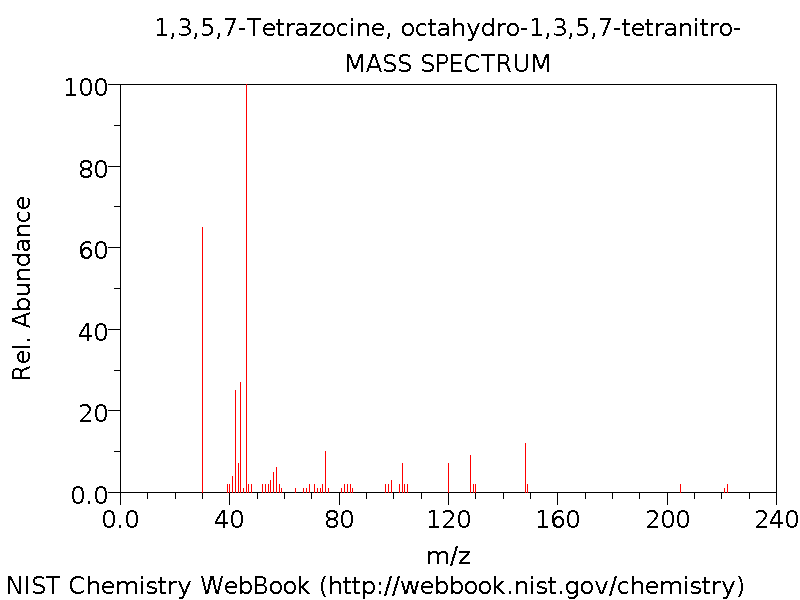
Mass Spectrum
Below is the observed mass spectrum[10] for HMX.
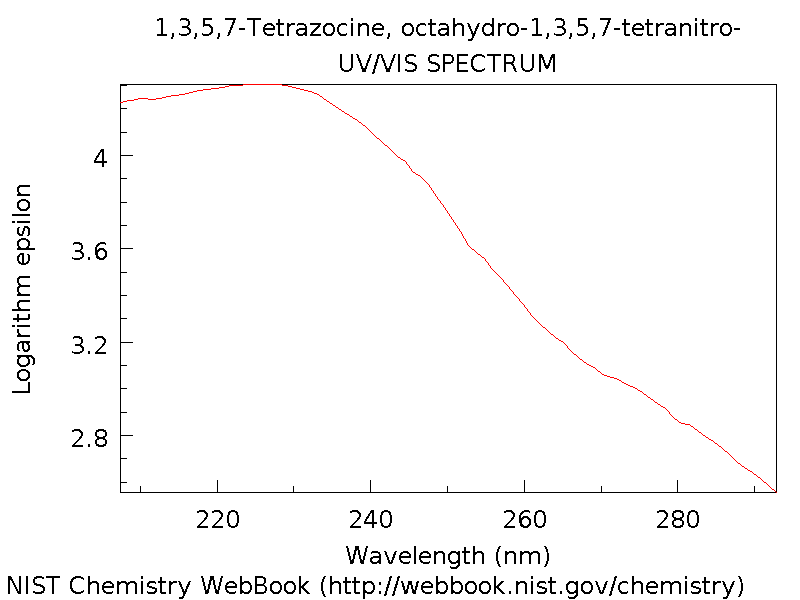
UV Spectrum
The UV spectrum[10] of HMX is shown below and gives a maximum at about 225 nm and the intensity drops significantly up to 290 nm. This makes sense as it is a colourless solid and thus exhibits very low absorption in the visible region.
1H NMR Spectrum
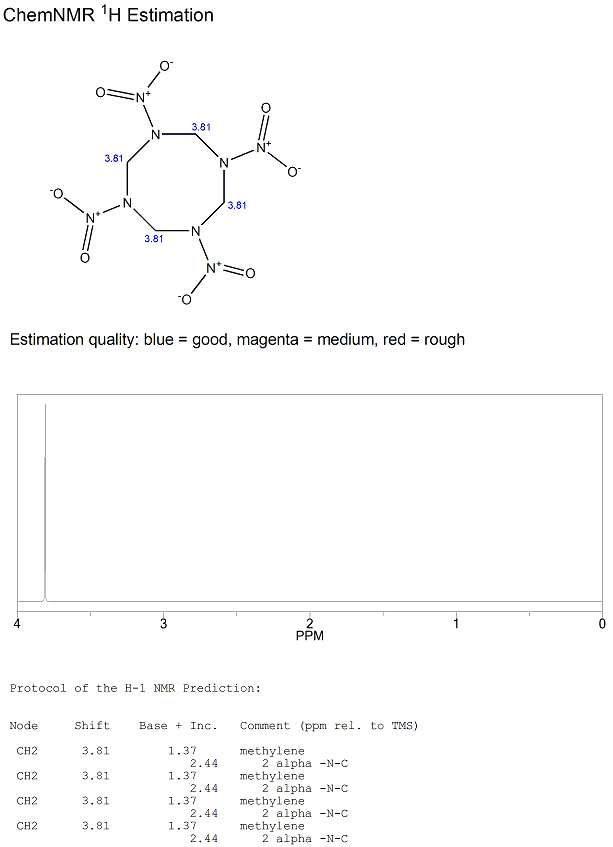
Like RDX, HMX gives a straightforward 1H-NMR spectrum with only one singlet. There are 4 methylenes, with 2 hydrogens on each. All the hydrogen atoms are both chemically and magnetically equivalent to each other so they do not couple with the hydrogens on the other methylenes. Hence, we only observe one singlet.
The predicted 1H-NMR spectrum using prediction software is shown below.
The software predicts a chemical shift of 3.81ppm for the singlet and this is in good agreement with the experimentally observed chemical shift[11] of 3.93ppm, which is recorded in DMSO solvent.
References
- ↑ 1.0 1.1 http://www.globalsecurity.org/military/systems/munitions/explosives-nitramines.htm
- ↑ http://en.wikipedia.org/wiki/HMX
- ↑ http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2003/hmx/History.htm
- ↑ Russian Journal of Organic Chemistry (Translation of Zhurnal Organicheskoi Khimii), 37(7), 1030-1033; 2001
- ↑ http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2003/hmx/Structure.htm
- ↑ http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2003/hmx/Properties.htm
- ↑ http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWebSites/2003/hmx/Uses.htm
- ↑ http://en.wikipedia.org/wiki/Octol
- ↑ http://en.wikipedia.org/wiki/OKFOL
- ↑ 10.0 10.1 http://webbook.nist.gov/cgi/cbook.cgi?ID=2691-41-0
- ↑ J. Chem. SOC. (B), 1968; 6-8