User talk:Rzepa1
Appearance
The use of Gaussian in an undergraduate computational laboratory: Spectroscopic simulations
- Henry Rzepa 07:06, 18 April 2012 (BST)
- Part of the NSCCS Applied Computational Chemistry Workshop for Synthetic Organic Chemists
- 17th & 18th April 2012, Imperial College London
- tinyurl.com/7x4vck8 /

The course essentials
- A five week course on molecular modelling comprising three modules
- ~organic, inorganic and physical chemistry
- Module 1 includes four set exercises ...
- Based on MM/QM
- and a QM-based mini-research-project
- in ~6 working days (but the lab is open 24/7!)
The Mini-Project in module 1
- A 1 week laboratory project for students to make a structural verification/correction based on calculations
- Each student has to browse the literature to ...
- identify a suitable (pair) of isomeric and ~rigid molecules ...
- of less than ~30 atoms (excluding H) to allow time for completion
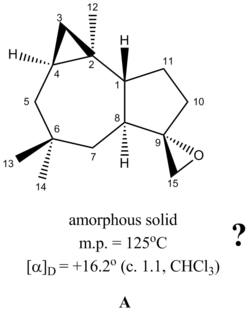
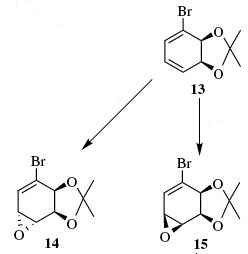
- Regio- and stereoselective conversion of alkenes to epoxides: Epoxides are highly versatile synthetic intermediates because they undergo ring opening with a wide range of nucleophiles. In a recent paper (DOI:10.1016/j.tetasy.2005.02.012 ), it was shown (Scheme 6 in the paper) that a 1,3-diene (compound 13) can be regioselectively epoxidized to give either stereoisomer 14 or 15 depending on the reaction conditions used. How would you tell that the “correct” alkene had been epoxidised? Unfortunately the authors don’t include 13C data for their products in the paper, but they are available in the literature (do a search on Beilstein). Do the products’ 13C data match your calculations? Why do the two sets of epoxidation conditions give different stereoisomers?
The protocol
- Draw the molecule using a sketching tool
- Students are reminded that drawing a bond does not mean the quantum mechanics will necessarily put one there
- Or, they could acquire coordinates by searching CCDC for their molecule (few students do this)
- The sketch is converted to realistic coordinates by interactive pre-minimisation with a mechanics force field (which preserves all drawn bonds!)
- And batch post-optimised using Gaussian at a medium basis set level and appropriate solvent (SCRF/CPCM).
- Alternative (open source) program: PSI4/Ψ4 which offers some very interesting features DOI:10.1002/wcms.93
- The final geometry is recovered (checkpoint file) and subjected to spectroscopic analysis (including solvent correction)
- NMR, including 1H, 13C, 31P, 15N, etc.
- OPTROT at specified frequency (default, 589nm) for optical rotation and ORD.
- TD-DFT for Electronic circular dichroism/UV-vis (nstates ≤ 20).
- FREQ(VCD) for (non-electronic) vibrational circular dichroism.
The Batch environment: SCAN
The real-time/batch decision moves each year.
- Currently, laptops can cope with ≤ 10 (non-H) atoms)
- Use of touch tablets/iPads for such a course?
- Computer room desktops with ≤ 15 (non-H atoms)
- more if students assiduously use symmetry, memory allocation, 4-processors.
- Batch for larger jobs, and including all FREQ(VCD) and nstates > 20.
- Research limit is currently triple-ζ/6-311G(d,p) basis for full QM on up to 250 atoms (inc. H)
- Up to about 200 atoms for full QM location of transition states
- Research limit is currently triple-ζ/6-311G(d,p) basis for full QM on up to 250 atoms (inc. H)
- Job submission is done via a HPC portal (Supercomputing@night: SCAN).
- Advantage that all resource allocations, processors etc are pre-selected.
- A standard script collects all relevant outputs for selection by students
- Digital repository deposition
- date stamps the calculation
- allocates a DOI
- which can be cited in e.g. a scientific publication or thesis
- The portal acts as an electronic laboratory notebook or ELN for comp chem.
- Synthetic chemistry is currently evaluating iLabber in a national trial and MNovaDB locally.
- Question: what is the most suitable e-labnotebook software for integrating compchem and synchem?
Interlude: a demonstration
- The SCAN portal
- The DSpace and Chempound data repositories
- The Figshare repository is coming!
The (student) Write-up
We use a (chemically enhanced) Wiki that includes the following features:
- Cloud-based, with full revision history and date stamps
- Simple templates for citations, DOI inclusion
- SVG support for scalable spectra produced by Gaussview
- Access to wiki commons image library, etc
- Full support for all advanced features of Jmol
- allows students' calculations to be included as active models in the report
|
|
Two examples of submitted coursework
Nick Mason: The structure and conformers of Stagonolide D
Alpha Lee: The Mystery of Epoxyafricane
- Follow the argument here
- See the conclusions
Conclusions
- Computational modelling increasingly integrated into both teaching and research labs
- Part of the future e-lab notebook?
- Consider the (chemical) Wiki for this task?
- Its publishing in journals is also evolving rapidly:
- The datument as a scientific article (or book)
- Digital data repositories (DOI:10.1021/ci7004737 ) are an essential component for modelling
- EPSRC research data policy framework