User:Yc14518
NH3 Molecule
Basic Information
| Categories | Information |
|---|---|
| Molecule | NH3 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -56.55776873 a.u. |
| RMS Gradient | 0.00000485 a.u. |
| Point Group | C3V |
Additional Information
| Categories | Information |
|---|---|
| N-H Bond Distance | 1.02Å |
| H-N-H Bond Angle | 106° |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986256D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol of NH3
NH3 Molecule |
Link to LOG File
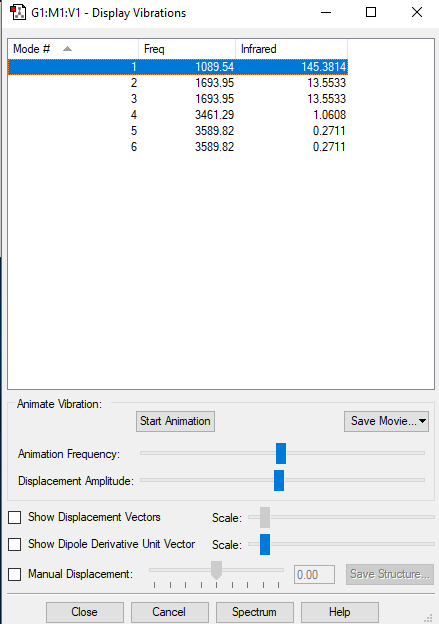
Display Vibrations
NH3 Vibrations
| Wavenumber cm^-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity Arbitrary Units | 145.4 | 13.6 | 13.6 | 1.1 | 0.3 | 0.3 |
| Image |  |
 |
 |
 |
 |

|
Answer Questions
how many modes do you expect from the 3N-6 rule?
- 3 x 4 - 6 = 6 modes I expected
which modes are degenerate (ie have the same energy)?
- Modes with wavenumbers of 1694 cm^-1 (number 2, 3) and 3590 cm^-1 (number5, 6) have the same energy.
which modes are "bending" vibrations and which are "bond stretch" vibrations?
- Modes with the wavenumber of 1090 cm^-1 (number 1) and 1694 cm^-1 (number 2, 3) are "bending" vibrations and modes with the wavenumber of 3461 cm^-1 (number 4) and 3590 cm^-1 (number 5, 6)are "bond stretch" vibrations.
which mode is highly symmetric?
- Mode with the wavenumber of 3461 cm^-1 (number 4) is highly symmetric.
one mode is known as the "umbrella" mode, which one is this?
- Mode with the wavenumber of 1090 cm^-1 (number 1) is the "umbrella" mode.
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
- Two bands since only modes with the wavenumber of 1090 cm^-1 and 1694 cm^-1 have dipole change when vibrate/stretch, and since modes with same wavenumber only show one peak.
Charge Analysis
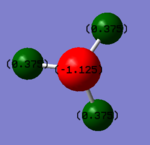
The charge of N is -1.125 and the charge of H is 0.375. N is expected to have negative charge and H is expected to have positive charge. This is because that N is more electronegative than H which pulls electric density to it and have negative charge.
N2 Molecule
Basic Information
| Categories | Information |
|---|---|
| Molecule | N2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -109.52412868 a.u. |
| RMS Gradient | 0.00000365 a.u. |
| Point Group | D∞h |
Additional Information
| Categories | Information |
|---|---|
| N-N Bond Distance | 1.11Å |
| N-N Bond Angle | 180° |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000002 0.001800 YES
RMS Displacement 0.000003 0.001200 YES
Predicted change in Energy=-1.248809D-11
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol of N2
N2 Molecule |
Link to LOG File
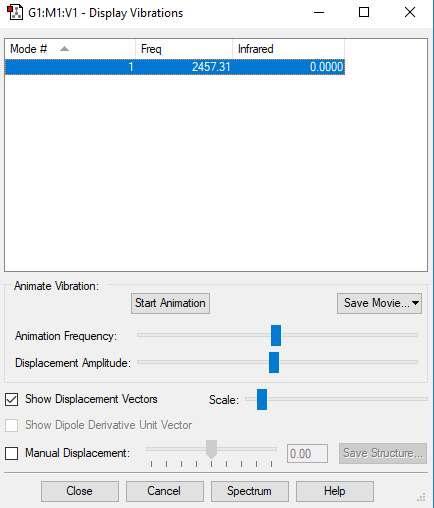
Display Vibrations
N2 Vibrations
| Wavenumber cm^-1 | 2457 |
| Symmetry | SGG |
| Intensity Arbitrary Units | 0.0 |
| Image | 
|
Charge Analysis
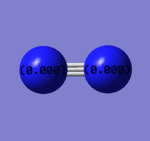
The charge of N is 0. N is expected to have zero charge since two atoms in the N2 molecule are same and the molecule vibrate symmetrically.
Computational and Experimental Comparison
I found (bis(2-(dicyclohexylphosphino)phenyl)(methyl)silyl)-dinitrogen-(trimethylphosphino)-cobalt structure [VEJSEL] and the two N-N distances are 1.10(5) and 1.13(1) Å. I think the difference between two values are caused by the reason that value calculated is under perfect condition which has no influence from other compounds or electrons. When N2 in this compound, CoN bond pulls part of electric density from NN bond which cause NN bond weaker and longer.
H2 Molecule
Basic Information
| Categories | Information |
|---|---|
| Molecule | H2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -1.17853930 a.u. |
| RMS Gradient | 0.00012170 a.u. |
| Point Group | D∞h |
Additional Information
| Categories | Information |
|---|---|
| H-H Bond Distance | 0.74Å |
| H-H Bond Angle | 180° |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000211 0.000450 YES
RMS Force 0.000211 0.000300 YES
Maximum Displacement 0.000278 0.001800 YES
RMS Displacement 0.000393 0.001200 YES
Predicted change in Energy=-5.852867D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7431 -DE/DX = -0.0002 !
--------------------------------------------------------------------------------
Jmol of H2
H2 Molecule |
Link to LOG File
Display Vibrations
H2 Vibrations
| Wavenumber cm^-1 | 4461 |
| Symmetry | SGG |
| Intensity Arbitrary Units | 0.0 |
| Image | 
|
Charge Analysis
The charge of H is 0. H is expected to have zero charge since two atoms in the H2 molecule are same and the molecule vibrate symmetrically.
Computational and Experimental Comparison
I found (Dihydrogen)-(1,2-ethanediamine-N,N'-dimethyl-N,N'-bis(2-benzenethiolato))-(tri-isopropylphosphine)-ruthenium structure [BIDQUB] and the two H-H distances are 0.74(3) and 0.91(2) Å. I think the difference between two values are caused by the reason that value calculated is under perfect condition which has no influence from other compounds or electrons.When H2 in this compound, RuH bond pulls part of electric density from HH bond which cause HH bond weaker and longer.
Energy for the Reaction
E(NH3)= -56.55777 au
2*E(NH3)= 2 x (-56.55777) = -113.11554 au
E(N2)= -109.52413 au
E(H2)= -1.17854 au
3*E(H2)= 3 x (-1.17854) = -3.53562 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -113.11554 - (-109.52413 - 3.53562) = -0.05579 au = -146.5 kJ/mol
-The ammonia product is more stable since this reaction is an exothermic reaction which indicated by negative ΔE value.
NF3 Molecule
Basic Information
| Categories | Information |
|---|---|
| Molecule | NF3 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -354.07131058 a.u. |
| RMS Gradient | 0.00010256 a.u. |
| Point Group | C3V |
Additional Information
| Categories | Information |
|---|---|
| N-F Bond Distance | 1.38Å |
| F-N-F Bond Angle | 102° |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000164 0.000450 YES
RMS Force 0.000108 0.000300 YES
Maximum Displacement 0.000612 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Predicted change in Energy=-1.274067D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.384 -DE/DX = 0.0 !
! R2 R(1,3) 1.384 -DE/DX = 0.0 !
! R3 R(1,4) 1.384 -DE/DX = 0.0 !
! A1 A(2,1,3) 101.8302 -DE/DX = 0.0001 !
! A2 A(2,1,4) 101.8302 -DE/DX = 0.0002 !
! A3 A(3,1,4) 101.8302 -DE/DX = 0.0002 !
! D1 D(2,1,4,3) 104.9443 -DE/DX = 0.0001 !
--------------------------------------------------------------------------------
Jmol of NF3
NF3 Molecule |
Link to LOG File
Display Vibrations
NF3 Vibrations
| Wavenumber cm^-1 | 482 | 482 | 644 | 930 | 930 | 1062 |
| Symmetry | E | E | A1 | E | E | A1 |
| Intensity Arbitrary Units | 0.5 | 0.5 | 2.8 | 208.1 | 208.1 | 39.9 |
| Image |  |
 |
 |
 |
 |
|
Charge Analysis
The charge of N is 0.660 and the charge of F is -0.220. F is expected to have negative charge and N is expected to have positive charge. This is because that F is more electronegative than N which pulls electric density to it and have negative charge.
NF3 MOs
| MO | MO1 | MO2 | MO3 | MO4 | MO5 |
| Graph | 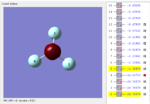 |
 |
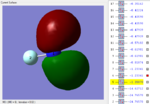 |
 |

|
Description:
MO1: N has a S orbital and deep in energy. It is not in the region of HOMO. S orbital here is the only one orbital so it is a nonbonding orbitals.
MO2: Here both N and F have S orbitals overlap toghther so they are bonding orbitals. They are not deep in energy and they are not HOMOs. Same as MO1, S orbital is the only type of MO present in this orbital. This MO is occupied.
MO3: In this orbital two F both have s orbital but in different phase, which means they are anti-bonding orbitals. They are not deep in energy and they are not HOMOs. Same as MO1 and MO2, S orbital takes 100 percent in all orbitals present. This MO is un occupied.
MO4: Under this condition,three F have three S orbitals; Two of the S orbitals are in the same phase and form a bonding orbital, and S orbital of another F is in anti phase and form an anti-bonding orbital with them. In this way, this MO is a mixture. Both of three orbitals are not deep in energy and they are not HOMOs. In addition, same as above, S orbital is the only type of orbital in this MO.
MO5: In this MO, P orbital is involved. N and one F (F2) have P orbitals and they are in the same phase that means they are bonding orbitals. Other two F (F1 and F3) both have S orbitals, and that two S orbitals are in the anti-phase with the P orbitals and form two anti-bonding orbitals. In this way, this MO is a mixture. In addition, this MO is not deep in energy and it is not a HOMO.
Independent work: CO2 Molecule
Basic Information
| Categories | Information |
|---|---|
| Molecule | CO2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -188.58093945 a.u. |
| RMS Gradient | 0.00001154 a.u. |
| Point Group | D∞h |
Additional Information
| Categories | Information |
|---|---|
| C-O Bond Distance | 1.17Å |
| O-C-O Bond Angle | 180° |
Item Table
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000017 0.000300 YES
Maximum Displacement 0.000021 0.001800 YES
RMS Displacement 0.000015 0.001200 YES
Predicted change in Energy=-5.259645D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1691 -DE/DX = 0.0 !
! R2 R(1,3) 1.1691 -DE/DX = 0.0 !
! A1 L(2,1,3,-2,-1) 180.0 -DE/DX = 0.0 !
! A2 L(2,1,3,-3,-2) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Jmol of CO2
CO2 Molecule |
Link to LOG File
Display Vibrations
CO2 Vibrations
| Wavenumber cm^-1 | 640 | 640 | 1372 | 2436 |
| Symmetry | PIU | PIU | SGG | SGU |
| Intensity Arbitrary Units | 30.7 | 30.7 | 0.0 | 545.8 |
| Image |  |
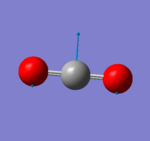 |
 |
|
Charge Analysis
The charge of C is 1.022 and the charge of O is -0.511. O is expected to have negative charge and C is expected to have positive charge. This is because that O is more electronegative than C which pulls electric density to it and have negative charge.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES - well done.
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - good explanations.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES
However you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 3/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - good explanation of the charges in terms of electronegativity.
You have worked hard on your MO explanation however it is difficult to follow. You have attempted to explain the AO contributions - this would be better if you specified 1s or 2s etc not just "S". Also each MO is an entire orbital it does not contain multiple bonding and antibonding orbitals. You could phrase this idea as bonding/antibonding interactions within the MO.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - you did an extra calculation with some good analysis well done!
Do some deeper analysis on your results so far