Third Year TS and Reactivity Lab: Exercises
Appearance
This is the assessed exercise section for the third year TS and reactivity lab. For information about the lab and assessment return to the main lab page. Make sure that you have completed the Tutorial section before attempting the exercises.
Exercise 1: Reaction of Butadiene with Ethylene
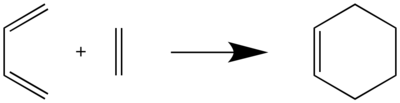
Calculations
- 1. Calculate the reactants, product, and TS at the PM6 level.
- 2. Run an IRC calculation to confirm that you have the correct TS.
- 3. Recalculate the reactants, products, and TS at the B3LYP/6-31G(d) level.
Write up and Analysis
Structure Analysis
- Confirm that you have the correct reactants, products, and TS. Explain how you can identify the nature of the stationary points on the PES. This only needs to be explained in detail for exercise 1, in the next two exercises your log files will be evidence that you know how to calculate and recognise the correct structures.
- Compare the results from the IRC calculation to the optimised butadiene. Are there any differences?
- Include measurements of the 4 C-C bond lengths of the reactants and the 6 C-C bond lengths of the TS and products.
- How do the bond lengths change as the reaction progresses? What are typical sp3 and sp2 C-C bond lengths? What is the Van der Waals radius of the C atom? How does this compare with the length of the partly formed C-C bonds in the TS? Is the formation of the two bonds synchronous or asynchronous (viewing the vibrational mode may help here too)?
MO Analysis
- Construct an MO diagram for the formation of the butadiene/ethene TS, including basic symmetry labels (symmetric/antisymmetric or s/a)
- View the MOs of the reactants and the TS in GaussView:
- Open the .chk (checkpoint) file of the structure.
- Under the Tools menu, choose MOs and visualise the MOs.
- Include images for each of the HOMO and LUMO of butadiene and ethylene, and the four MOs these produce for the TS on your MO diagram with the corresponding MOs in your MO diagram.
- What can you conclude about the requirements for symmetry for a reaction (when is a reaction 'allowed' and when is it 'forbidden')? Write whether the orbital overlap integral is zero or non-zero for the case of a symmetric-antisymmetric interaction, a symmetric-symmetric interaction, and an antisymmetric-antisymmetric interaction.
Method Comparison
- Calculate the activation enthalpies at both the PM6 and B3LYP/6-31G(d) levels of theory.
- What is the difference in the activation enthalpies between the methods?
- It is hard to find accurate experimental barriers for the reaction, one high-temperature experimental activation enthalpy is 27.5 kcalmol-1 (760-920 K) [[1]]. High-level computational chemistry calculations have been carried out on the reaction and one activation enthalpy (298.15K; MRAQCC calculation) was calculated at: 23.14 kcalmol-1[[2]]. How do your activation enthalpies compare? What can be evaluated about the methods (PM6 or B3LYP/6-31G(d)) and which method did you expect to be more accurate? What problems or errors might there be comparing your calculated values to the experimental value?
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
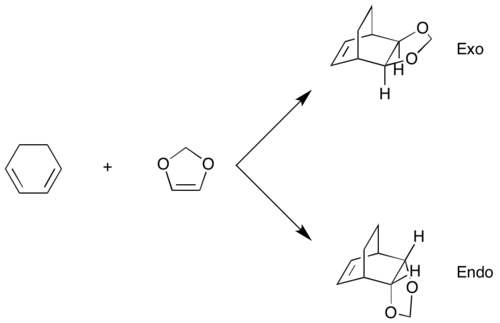
Calculations
- 1. Calculate both the endo and exo TSs at the PM6 level.
- 2. Calculate the reactants: cyclohexadiene, 1,3-dioxole, and both the endo and exo products at the PM6 level.
Write up and Analysis
MO Analysis
- View the MOs of the structures and use your MO diagram for the Diels-Alder reaction to locate the occupied and unoccupied orbitals associated with the DA reaction for both TSs by symmetry.
- Construct a new MO diagram using these new orbitals and adjust the energy levels as necessary.
- Is this a normal or inverse demand DA reaction?
- Analyse the HOMO of both the endo and exo TS structures. Are there any secondary orbital interactions or sterics that might affect the reaction barrier energy? (Hint: in GaussView, set the isovalue to 0.01 when viewing the HOMOs).
The Wikipedia page on Frontier Molecular Orbital Theory has some useful information on what these secondary orbital interactions are.
Reaction Barriers
- Tabulate the free energies (kJmol-1; room temperature) of the reactants, TSs and products.
- Calculate the reaction barriers and reaction energies (kJmol-1; room temperature).
- What are the kinetically and thermodynamically favourable products?
Exercise 3: Diels-Alder vs Cheletropic
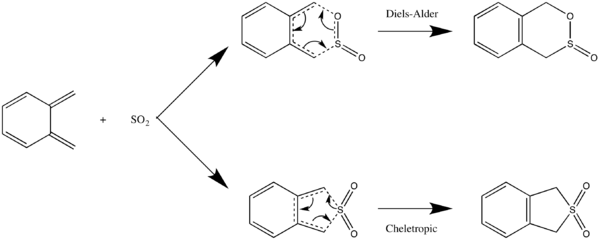
Calculations
In the tutorial, you will have ended up with either the endo or the exo TS and adduct for the Diels-Alder reaction.
- 1. Calculate the other TS and adduct (exo or endo) at the PM6 level.
- 2. Calculate the chelotropic TS and adduct at the PM6 level.
- 3. Calculate the reactants at the PM6 level.
- 4. Run IRC calculations for the endo, exo Diels Alder, and the chelotropic TS structures.
You should now have TSs and products for each of the cheletropic, and the endo and exo Diels-Alder reactions
Write up and Analysis
Reaction Barriers
- Calculate the free energy reaction barriers and reaction energies (room temperature; kJmol-1) for each of the three reaction pathways.
- Determine and explain which reaction pathway is preferred.
Reaction Profile
- Construct an annotated reaction profile for the three pathways (it is suggested to use Excel/ChemDraw)
- The reaction profile must use the relative energies of the reactants, TSs, and products. The energy level of the reactants at infinite separation can be used as the 0 energy level.
Mechanism
- View the IRC calculations for the reactions.
- Xylylene is highly unstable - what happens to the bonding of the 6-membered ring during the course of the reaction?
- What determines whether the reaction follows the Diels-Alder endo or exo, or the chelotropic mechanism?
