StudentWiki:HaloalkanesAlcoholsAmines
Haloalkanes, Alcohols, Amines
Nomenclature
Haloalkanes
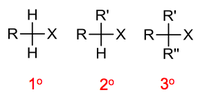
Depending on the substitution of the carbon bearing the halogen atom, we classify alkyl halides as primary (1°), secondary (2°) or tertiary (3°).
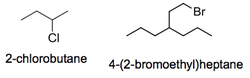
The naming of haloalkanes follows these general rules:
- Find the longest carbon chain (even if it does not contain the halogen)
- Use lowest possible numbers for the position of any branched alkyl chains or for the halogen itself
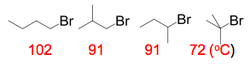
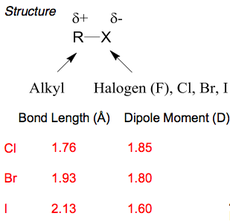
Haloalkanes have higher boiling points than the corresponding alkanes. This is due to the increased size of the halogen in the molecule that increases the Van der Waal forces present within the bulk material, thereby requiring more energy to boil. Additionally, the boiling point increases for homologous series, also due to the increasing halogen size: Cl < Br < I.
Increased amounts of branching in the molecules also reduces the boiling point, due a decrease in density since the molecules can no longer lie almost linearly next to each other. Thus reduces the amount of Van der Waal's forces acting between the molecule, hence reducing the input energy required for boiling.
Cl, Br and I-haloalkanes are more dense than water.
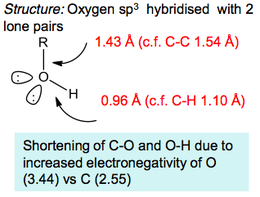
Alcohols
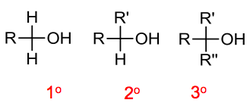
In the same way as for haloalkanes we classify alcohols as primary (1°), secondary (2°) or tertiary (3°).
The naming of alcohols follows these rules:
- Find the longest carbon chain containing the alcohol functional group.
- Number the carbons starting from the alcohol group.
- Number and name all substituents.
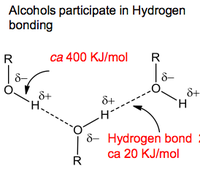
Alcohols have an important feature, which is that they can undergo hydrogen bonding to other alcohol molecules.
Even though the hydrogen bonding isn't particularly significant, it is enough to push their boiling points up much higher than any analogous alkanes. Hydrogen bonding is also responsible for the ease at which small molecular weight alcohols dissolve in water (although as the hydrocarbon chain on alcohols increases, the non polar properties increase and dominate the polar properties of the -OH group).
Amines
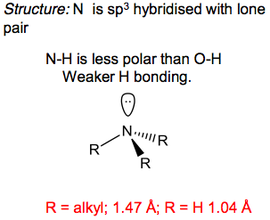
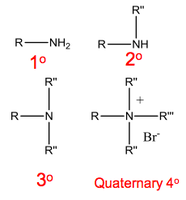
Amines are classified according to the number of carbon atoms bound to the nitrogen.
The naming of amines follows these general rules:
- A primary amine is considered as a simple functional group like an alcohol group.
This means that the longest carbon chain is considered the principal name of the molecule, whether is contains the amine group or not.
- A secondary (2°) or tertiary (3°) amine, however, considers the longest alkyl chain attached to the N to be the principal name of the molecule.
Any additional substituents are names with an N- prefix.
Amines are very distinct in that they have a fairly strong "fishy" smell, and for example 1.5-pentanediamine smells of rotting flesh (giving the molecule's alternative name of ‘cadavarene’). In the same that alcohols had higher than expected boiling points, amine have high boiling points with respect to their analogous hydrocarbons thanks to hydrogen bonding. It's also responsible once again for the solubility of low molecular weight amines in water. Primary and secondary amines can be H-bonding donors, and tertiary ones are only H-bonding acceptors.
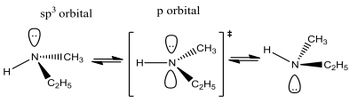
Amines have a typical trigonal pyramidal structure thanks to the nitrogen's lone pair repelling the three N-C bonds away from itself. This structure exists in an equilibrium state of interconversion, where the transition state is in fact a pseudo-tripgonal bipyramidal structure. This is due to the order of hybridisation changing on the nitrogen. When it's trigonal pyramidal, the nitrogen is sp3 hybridised. The lone pair also exists in an sp3 orbital, which gives the molecule a pseudo-tetrahedral structure (check out the VSEPR section in Molecular Structure). In the transition state, the nitrogen is sp2 hybridised, which means there is a free p-orbital in which the lone pair sits.
Reactivity
Haloalkanes
Haloalkanes are good electrophiles for nucleophilic substitution (where the C-X bond would be broken).
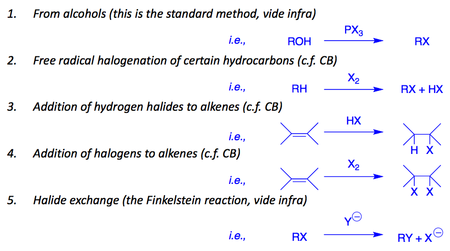
Here is an overview of their preparation.
Alcohols
Alcohols are only relatively weak electrophiles, but when deprotonated are good nucleophiles and good bases.
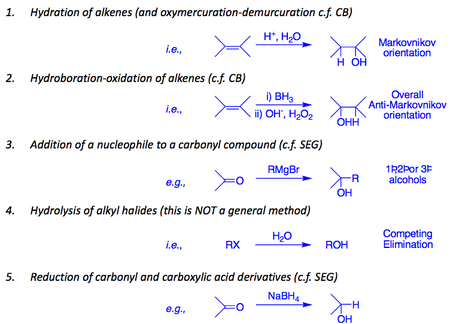
Here is an overview of their preparation.
Amines
The lone pair on amines renders them good nucleophiles and good bases.
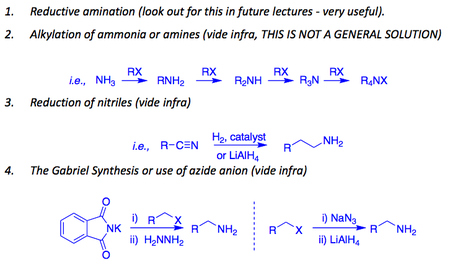
Here is an overview of aliphatic amine preparation.
Nucleophilic Substitution in Alkyl Halides
Nucleophilic substitution has already been covered in depth with respect to carbonyls in the Carbonyls and Carboxyls course. With carbonyls, nucleophilic substitution proceeds via an attack of the nucleophile on the π* orbital of the carbonyl bond. The population of thus MO leads to a reduction in bonding order, with the localisation of the charge on the oxygen atom. This charge is then free to reform a double bond, with the release of a good leaving group.
However, for alkyl halides this mechanism does not take place because of the lack of double bond and anitbonding π* orbital.
Sir Christopher Ingold investigated the kinetics, and hence mechanism by which the simple hydrolysis of haloalkanes takes place.
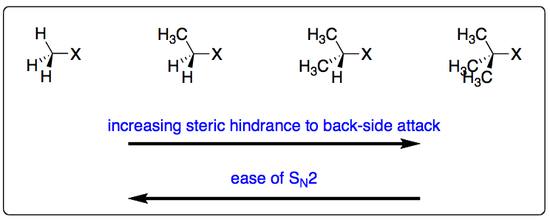
He found that for a tertiary haloalkane, the rate of reaction was first order with respect to the haloalkane. A primary haloalkane, on the other hand, gava a rate of reaction that was dependent not only on the haloalkane but also on the concentration of hydroxide ion, making it overall second order. This lead to the conclusion that two different mechanisms must be operating:
- The SN1 reaction for the tertiary haloakane.
- The SN2 reaction for the primary haloalkane.
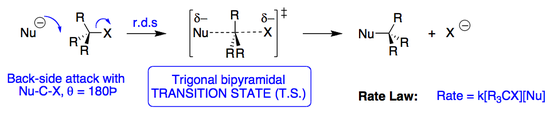
SN2 Reactions
An SN2 reaction is a nucleophilic substitution, where:
- S = substitution
- N = nucleophilic
- 2 = the rate determining step (RDS) is bimolecular.
The reaction proceeds via a single intermediate, and with a complete inversion of stereochemistry at the end of the reaction.
The rate shows that the RDS is dependent on both the alkyl halide and the nucleophile.
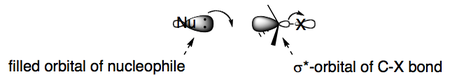
A filled orbital on the nucleophile attacks the empty σ* orbital of the C-X bond, thereby reducing its bond order to zero.
The best angle for approach is at 180° due to maximum orbital overlap. This is called a "backside attack".
An SN2 reaction is a concerted reaction, with only a transition step (energy maximum) and no intermediates (energy minimum).
The reaction coordinate of the reaction is displayed below. Because the RDS involved the simultaneous association of the nucleophile and part-dissociation of the halogen, the transition state has a trigonal bipyramidal structure, with a loss in polarity
A polar aprotic solvent is best for carrying out an SN2 reaction.