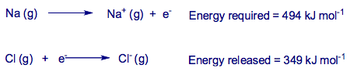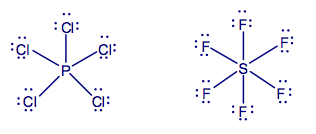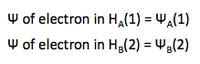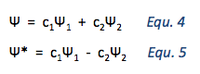StudentWiki:MolecularStructure
Molecular Structure
Bond Types and Valence Electrons
CHEMICAL BOND
A chemical bond is a link between atoms. It forms if the resulting arrangement of the
two nuclei and their electrons has a lower energy than the total energy of the separate atoms.
IONIC BOND
When the complete transfer of one or more electrons from one atom to another takes place,
generating charged species that are held together by electrostatic interactions. For example:
In the case of NaCl, there is apparently no inducement for the ionic solid to form due to the large energy demand in making Na+ ions.
However, the formation of NaCl is in fact favourable due to the highly strong electrostatic forces between chlorine and sodium atoms.
Taking this into account, the net change in energy for the formation of NaCl is: -642 kJ/mol, an endothermic process.
METALLIC BONDS
In this type of bond, a lorge number of cations are held together by a "sea" of delocalised electrons.
For example, a piece of copper consists of a stack of copper ions all held together by the electrostatic force of a "sea" of electrons,
each of which comes from a single atom in the sample. This "sea" of delocalised electrons is one of the explanations as to why
metal cutlery, for example, feels cold - the delocalised electrons are easily capable of conducting heat and electricity
(the electrons are easily able to jump to and from different energy states).
COVALENT BONDS
A covalent bond between two atoms forms when the atoms share electrons to achieve the lowest possible energy arrangement.
Most non-metallic elements in the periodic table exist as molecules (not as free atoms).
In these molecules, two or more atoms share their electrons to form metal bonds.
Summary
The way in which atoms are linked together is essential in defining the properties of substances. When studying and understanding molecules, the geometry and stucture of the molecule and its bonding is key. In other words, it can be said that the changes in energy associated with the formation of bonds are a consequence of the "relocation" of the valence electrons. Valence electrons are the electrons in the outermost shells of an atom. They can be either donated from one atom to another (ionic), shared between two atoms (covalent) or be part of a larger cloud of electrons that holds cations together (metallic).
Although it is useful to classify bonds as ionic, covalent or metallic, it is important to note that chemical bonding in real molecules and compounds must be considered as the sum of more than one type of bond. For example, in the diatomic molecule H2, there is a "pure" covalent bond that exists between the individual atoms since the electrons are shared equally. In hydrogen fluoride, the covalent bond is polarised, with the greater electron density lying towards the fluorine atom, due to its higher electron affinity and electronegativity. In a molecule such as this one, the bond has both covalent and ionic character.
Chemists apply models and theories in order to explain the structure of molecules and their behaviour. Some theories explain a better specific phenomenon than others and certain problems can be better understood using one model rather than another. Nevertheless, all models have certain restrictions and limitations, which will be illustrated within the next section.
Lewis Structure
Our current models and theories of chemical bonding stem from the work of G. N. Lewis, dating from 1916 (which you can access here: DOI:10.1021/ja02261a002 ). Lewis proposed the explanation that covalent bonds implicate a sharing of electrons. He was the first scientist to put forward a sensible explanation for chemical bonding, a phenomenon that had baffled many scientists previously.
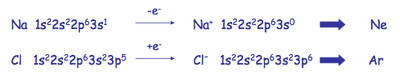
In general, the formation of an ionic compound involves the loss and gain of electrons from one atom to the other, so that both atoms eventually obtain a "Noble Gas Configuration" whereby their octets would be complete. Using NaCl as an example:
Lewis extended this idea to the covalent bond and called the principle the Octet Rule. In covalent bond formation, atoms go as far as possible towards completing their octets by sharing electron pairs.
LEWIS SYMBOLS
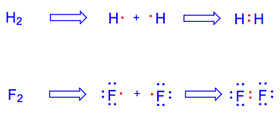
Lewis devised a simple way of representing electrons in an atom to keep them when bonds form. He represented each valence electron as a dot, and arranged the dots around the symbol of the element. A single dot represents an electron alone in an orbital, while a pair of dots represent two paired electrons sharing an orbital.
Thus, a picture of bonding starts to form:
Constructing Lewis Structures
| STEP 1 | Count the number of valence electrons on each atom - for ions, adjust the number of electrons to account for the charge. Divide the total number of electrons by 2 to obtain the number of electron pairs. | |
| STEP 2 | Decide on the most likely arrangement of atoms, choosing which one goes at the "centre" of the molecule (usually the least electronegative, although there are several exceptions). | |
| STEP 3 | Place one electron pair between each pair of bonded atoms. | |
| STEP 4 | Complete the octet of each atom (or duplet for H and He) by placing any remaining electrons around the atoms. If there are not enough electron pairs, from multiple bonds. | |
| STEP 5 | Represent each bonded electron pair by a line. |
A couple of examples are given below:
Resonance
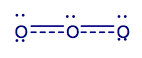
One of the limitations of the Lewis model is that some molecules cannot be respresented by a single Lewis structure. A classic example is the structure of ozone, O3. The Lewis structure suggests two different O-O bonds within the molecule - one single bond (148 pm) and one double bond (121 pm)- whereas experimental data shows that only one bond length is found (128 pm). Thus, the Lewis model does not explain the experimental data!
Superposition of all possible Lewis structures represents the real bonding situation (resonance is indicated by a double-headed arrow). This superposition has to be considered as a "blending" rather than a flickering alteration between the different forms. The total sum of all Lewis structures is called a resonance hybrid and a single structure within the resonant forms is called a canonical structure. The resonance hybrid of ozone is given below. It has a total bond order of 1.5 instead of 1 and 2 combined.
It is important to note that resonance only describes the varying locations of electrons around the molecules.
Different atom arrangements is known as isomerism. They are both very different things!
Hypervalence
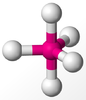
Lewis theory based on the octet rule holds well for most molecules with central atoms of the second period in the Periodic Table. However, in the case of heavier elements, the octet may be exceeded. For example: PCl5 (10e), SF6 (12e). This is known as hypervalence.
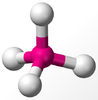
VSEPR Theory
The structure (or shape) of molecules is important with respect the a compound's physical and chemical properties. A good example is the massive difference in smells between the two enantiomers (S)-carvone and (R)-carvone, where simply the orientation of a two groups changes everything.
The Lewis structures discussed so far are 2D representations of the bonds between atoms. This doesn't give much indications as the the actual arrangement of the bonds and atoms in space, i.e. the geometry of the molecule. The Valence-Shell Electron Pair Repulsion model extends Lewis' theory of bonding to account for molecular 3D shape. It works by considering the electron pair repulsions of both bonding pairs and lone pairs, which in turn leads to defining bond angles in molecules.
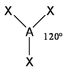
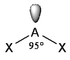
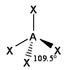
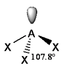
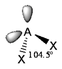
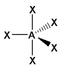
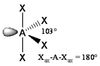
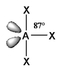
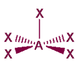
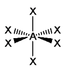
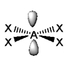
The basic concepts within VSEPR theory is that for a molecule of general formula AXnEm, all ligands (X) and lone pairs (E) around a central atom (A) adopt positions as far apart from each other as possible to minimise electrostatic repulsions. In order to predict the geometry of a molecule using this model, its Lewis structure needs to be determined first. With this, it is possible to identify all bonding electron pairs (BP) and lone pairs of electrons (LP).
The key points to know when dealing with electron pair repulsions are the following:
- Repulsions have an order of strength: LP-LP > LP-BP > BP-BP
- LPs occupy the largest site (e.g. equatorial in trigonal bipyramidal)
- If all site are equal, the LPs will be -trans to each other.
- Double bonds occupy more space than single ones.
- Bonding pairs to electronegative substituents occupy less space than those to electropositive ones.
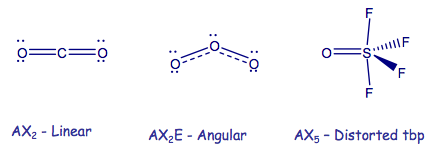
AXnEm Structure Predictions
| STEP 1 | Count electron pairs, both BP and LP by determining the Lewis structure of the compound. | |
| STEP 2 | Find the structure that minimises electron repulsions by using the criteria listed above. | |
| STEP 3 | Determine the pseudostructure (arrangement of BP and LP) and then the actual structure (arrangement of atoms). |
In VSEPR, there is no need to distinguish between single and multiple bonds: a multiple bond us treated as a single region of high electron concentration. Two electrons in a double bond "act together" when we take into account their repulsion with respect to other regions of charge density. Nevertheless, double bonds do have a large volume of charge density and therefore "push" other single bonds more strongly away from it than a single bond would. Examples:
Limitations of VSEPR
The main limitations of VSEPR to be concerned with are the following:
- If energy differences between possible shapes become small, VSEPR often fails to predict the true geometry.
- VSEPR predicts wrong structures for molecules of heavy atoms. In these cases, the LPs are sterically non-active.
- VSEPR does not apply to Transition Metal compounds
Symmetry and Point Groups
Symmetry
A molecules displays symmetry if you can rotate/reflect/translate it so that it appears unchanged. Symmetry is found i a massive amountof everyday objects, but in chemistry and for molecules, symmetry is needed for:
- Chemical bonding - molecular orbitals (MO) can only form if the atomic orbitals (AO) have the same symmetry and similar energy.
- Spectroscopy - symmetry of the molecule influences its interactions with electromagnetic radiation.
- Physical Properties - e.g. melting and boiling points, polarity, etc..
SYMMETRY ELEMENT - a point, line or plane to which the symmetry operation is performed.
SYMMETRY OPERATION - an action (rotation, reflection, etc) that moves a molecule into a new orientation equivalent to its original one.
| Symbol | Element | Operation |
|---|---|---|
| E | Identity Element | Leaves the object unchanged; it's like a rotation of 360°. Any molecule, even a complex protein has E. |
| Cn | Rotational Axis | Rotation about the axis by 360/n, where n is an integer. When a molecule has several rotational axis, the one with the greatest n is the Principal Axis, and is usually about the z-axis. |
| σ | Plane of Symmetry | Reflection in plane of symmetry. There are three types to distinguish: σv: mirror plane parallel to principal axis. σh: plane perpendicular to principal axis. σd: plane bisects angle between two rotational axes. |
| i | Centre of Inversion | Projection through the centre of inversion to equal distance on the other side. Inversion is not necessarily the same as a rotation about C2 |
| Sn | Improper Rotation Axis | Rotation by 360/n, followed by reflection in plane perpendicular to the rotation axis. Interestingly, neither the plane nor that rotational axis need to be part of the molecule's symmetry. |
Point Groups
Point groups describe a full set of symmetry operations, and are the most accurate means of describing and labelling a molecule, i.e. D4h is even more accurate than just "square planar" as a name.
Assigning the highest order rotational axis (the Principal Rotational axis) is usually the most important step when finding the point group of a molecule, and there is a systematic method that has been developed for the determination of the point group. This method is summarised in the flow chart given below:
Valence Bond Theory
Valence Bond Theory (VB Theory) was the start of a quantum mechanical explanation for chemical bonding. It began in 1927 with a paper put forward by W. Heitler and F. London, and was further developed by L. Pauling, J. C. Slater and C. A. Coulson. It can be regarded as a way of describing Lewis Theory in terms of wavefunctions, i.e. treating the electrons as waves in addition to their being particles.
The basic idea behind valence bond theory is that discrete atoms with one or more unpaired electrons form a bond by the overlapping of atomic orbitals. This process is accompanied by the pairing of electron spins, which is close to the idea of Lewis H-H bonds.
Description of H2 using VB Theory
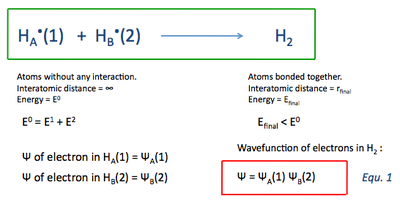
STARTING POINT
Two discrete H atoms (A and B) with one electron each (1 and 2) are far away from each other (or in other words, at infinity from each other). In this situation there is no interaction/perturbation between the two atoms, which can be expressed by the following wavefunctions:
BOND FORMATION
The atoms then start approaching each other, forming a bond and instantly generating a perturbation of the total energy of the separated atoms. For bond formation to take place, the final energy of the molecule must be smaller than the total energy of the two separate atoms. If the change in energy, ΔE, is lower than zero, the bond formation process is exothermic. If it is greater than zero, it is endothermic.
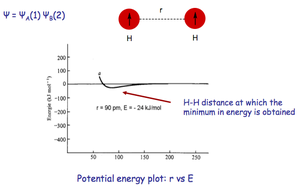
The graph below is a plot of the total potential energy of a system containing HA(1) and HB(2). The wavefunction of the bond, Ψ, is a combination of the wavefunctions of electrons 1 and 2, Ψ1 and Ψ2. Function Ψ is dependent on the interatomic distance, r, of the two atoms, and when it reaches an energy minimum a bond exists. This energy minimum is the point at which electron-electron repulsion and electron-nucleus attractions are at a perfect equilibrium.
However, this understanding of the wavefunction of electrons within the bond is not sufficient, die to the following observations:
| Experimental data : | Approximation represented by the wavefunction : | |
| * bond distance = 74 pm | * bond bond distance = 90 pm | |
| * ΔE = -458 kJmol-1 | * ΔE = -24 kJmol-1 |
The problem arises in the fact that so far we have assumed that electron 1 always stays with HA and electron 2 always with HB.
This is true for discrete atoms, but not for a molecule. For molecules, it is not possible to assign any particular electron to a specific nucleus. Therefore, the wavefunction must be extended to take into account the electron exchange that takes place between the nuclei.
The H2 molecule is thus described by a combination of the equations 2 and 3.
More accurately, this can be represented as:
Although the values of c1 and c2 are the same in the case of the symmetric molecule H2, there are two different possible linear combinations:
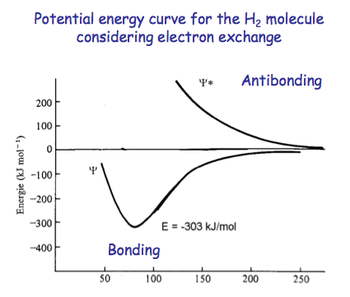
The first of these combinations refers to the spin pairing of electrons, leading to a bonding situation and a decrease in energy.
The second of these combinations refers to parallel electron spins, leading to a repulsive, antibonding state with higher energy.
Considering electron exchange, we can see that the potential energy curve for the H2 molecule consists of two parts.
Further improvements can me made by taking into account:
- Variations in the effective nuclear charge - each electron is now attracted to two nuclei instead of just one, so Zeff
- Polarisation - electron cloud is distorted towards the bonding region of the molecule
- Ionic contributions - we consider a situation in which both electrons are placed in one of the two H atoms (through resonance).