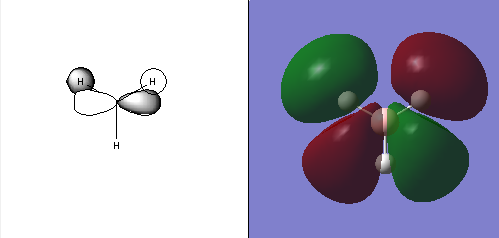Rep:Title=Mod:qaa12
EX3 section
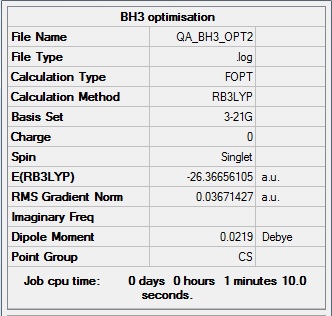
BH3 section
B3LYP/3-21G level
Optimisation log file here
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
B3LYP/6-31G(d,p) level
Optimisation log file here
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
GaBr3 section
B3LYP/LANL2DZ level
Optimisation log file DOI:10042/85015
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
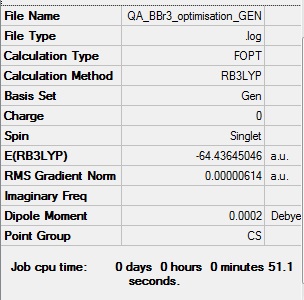
BBr3 section
B3LYP/GEN level
Optimisation log file DOI:10042/85082
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
Geometry Data Comparison
| BH3 | BBr3 | GaBr3 | |
|---|---|---|---|
| r(E-X) | 1.19Å | 2.35Å | 1.93Å |
| θ(X-E-X) | 120.0° | 120.0° | 120.0° |
From the optimisations of BH3, BBr3, and GaBr3, it was found that the average bond length, r(E-X), was 1.19Å in
B-H, 2.35Å in B-Br, and 1.93Å in Ga-Br. The average bond angles were all 120.0° for each of the three molecules as shown in the table above.
Comparing BH3 and BBr3, changing the ligand from H to Br had a very large increase in bond length with an increase of approximately 1.15Å. Both H and Br have 1 valence electron available for bonding and hence forms a single bond with Boron. However, Br is a much larger atom than H with more electrons and larger 4p valence electron orbital used in bonding as compared to hydrogen, which only have a small, closely-held 1s orbital. Therefore, the bond formed in B-Br is much longer than that in B-H, but their bond angles remain equal. Also, Br is much more electronegative than H and it pulls/distorts electron orbitals towards itself.
Comparing BBr3 and GaBr3, whereby the central atom was changed from B to Ga, the bond angle remains the same but the bond lengths decreases from B to Ga. Although Ga is a larger atom than B (Ga has larger 4p orbitals compared to smaller 2p orbitals in B), The Ga-Br bonds formed were shorter because of better orbital overlap between the Ga and Br due to their orbitals being closer in energy and similar radii as compared to that in B-Br, where the overlap is relatively poor. Therefore, the bond formed in Ga-Br is noticeably shorter than that in B-Br.
According to the Valence Bond Theory, a chemical bond is formed from the constructive interference of an electron in an atomic orbital of a particular atom pairs up its spin with the spin of another electron in another atomic orbital of another atom [7]. The linear combination of the two wave functions of each atomic orbital results in a new wave function formed which is lower in energy (bonding orbital) than either separate atoms.
psi = A(1)B(2) + A(2)B(1)
where 1 = electron 1, 2= electron 2 A and B are separate individual atoms.
The destructive interference however, results in a higher energy anti-bonding orbital. If the anti-bonding orbital is filled with electrons, the bond order is reduced. In the Molecular Orbital Theory, the electrons are not fixed to individual bonds. Instead, they are spread out through the entire molecule.
Some examples of strong bonds are ionic bonds. For example, the lattice energy of NaCl is -786 kJ/mol.[1] Covalent bonds, however, vary greatly in strength in terms of their bond dissociation enthalpies. An example of a strong diatomic covalenlt bond would be the triple N≡N bond in N2 which has an energy of 945 kJ/mol [5]. A medium bond would be the C-C single bond with bond dissociation energy of 348 kJ/mol [5]. An example of weak bonds can be hydrogen bonds with bond energy of approximately 3-32 kJ/mol [6].
BH3 B3LYP/6-31G(d,p) Frequency
Frequency log file here
| Summary Data | Low frequencies |
|---|---|
 |

|
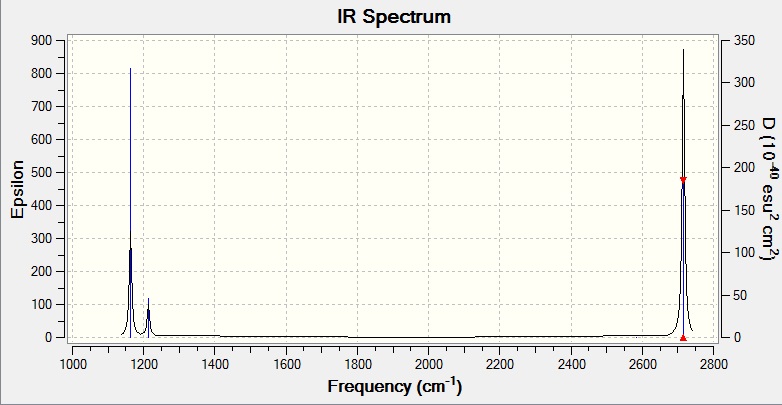
Vibrational Spectrum for BH3
| Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|
| 1163 | 93 | Yes | Bend |
| 1213 | 14 | Very slight | Bend |
| 1213 | 14 | Very slight | Bend |
| 2583 | 0 | No | Stretch |
| 2716 | 126 | Yes | Stretch |
| 2716 | 126 | Yes | Stretch |
Although there are 6 vibrational modes, only 5 of them are IR active. For a vibrational mode to be IR active, the vibration must first result in a net change in dipole moment within the moleucle. The symmetric stretch at 2583 cm-1 is not IR active and would not show on the IR spectrum. Only 3 peaks appear because the two asymmetric stretches at 2716 cm-1 are degenerate and the 2 weak bends at 1213 cm-1 are also degenerate. Hence they appear as single peaks respectively. As a result, only 3 peaks appear.
GaBr3 Frequency
Frequency log file DOI:10042/90214
| Summary Data | Low frequencies |
|---|---|
 |
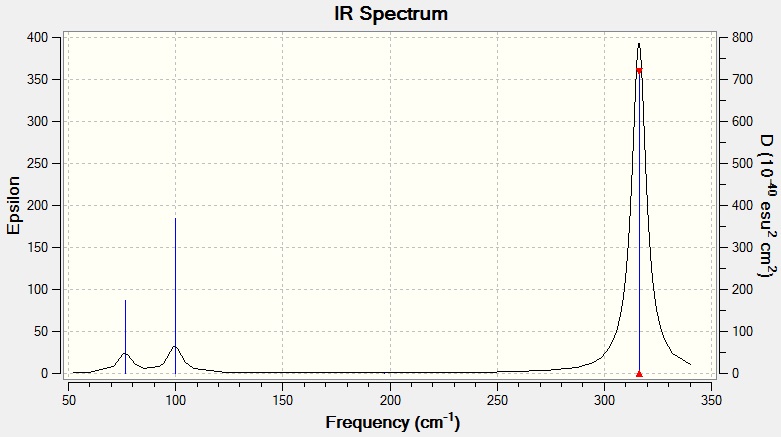
Vibrational Spectrum for GaBr3
| Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|
| 76 | 3 | Very slight | Bend |
| 76 | 3 | Very slight | Bend |
| 100 | 9 | Very slight | Bend |
| 197 | 0 | No | Stretch |
| 316 | 57 | Yes | Stretch |
| 316 | 57 | Yes | Stretch |
now compare and contrast the vibrational spectra for BH3, and GaBr3. (this analysis should be 2-3 paragraphs long.) Some leading questions you should consider are:
What does the large difference in the value of the frequencies for BH3 compared to GaBr3 indicate?
Both BH3 and GaBr3 have the same types of vibrational modes (2 degenerate asymmetric stretches, 1 symmetric stretch, 1 umbrella bend, 2 degenerate asymmetric bending). However, BH3 have vibrational frequencies of much higher magnitude in terms of wavenumbers than GaBr3.
The large difference in the frequencies for BH3 and GaBr3 represents the difference in energy of the vibrations within the molecules. The vibrational energy is related to frequency according to:
where v is the frequency of the vibration in wavenumbers. According to the equation, the higher frequencies in BH3 represent much higher energy vibrations than that in GaBr3. The vibrational energy of GaBr3 is much lower because of the much heavier atoms Ga and Br compared to B and H.
The relationship between atomic mass and vibrational frequency is given by the equation shown below:
Compare the relative frequency and intensity of the umbrella motion for the two molecules. Looking at the displacement vectors how has the nature of the vibration changed? Why?
From the displacement of the umbrella motion of BH3, the lighter H atoms have a larger and pronounced movement than the relatively heavier B atoms. The opposite is true for the umbrella motion of the GaBr3 molecule in which the central Ga atom (being lighter) have the larger movement than the heavier Br atoms.
Why must you use the same method and basis set for both the optimisation and frequency analysis calculations?
Using the same basis set ensures consistency in the calculations for the optimisation and frequency analysis.
What is the purpose of carrying out a frequency analysis?
The frequency analysis allows us to identify vibrational modes that are IR inactive and otherwise would not be detected by IR spectroscopy. It also enables us to visualise the type of vibrations that occur. A frequency analysis also enables us to calculate the energies of the bond and hence its strength.
What do the "Low frequencies" represent?
The "low frequencies" represent the translational movement of the molecule through space along the (x,y,z) axes. The frequencies associated with such movements were disregarded as they are very low in energy relative to the vibrational movements within the molecule. As such, the molecules were considered to be "fixed" in space.
BH3 MO Energies
Energy .fch file DOI:10042/90254
ChemDraw file here
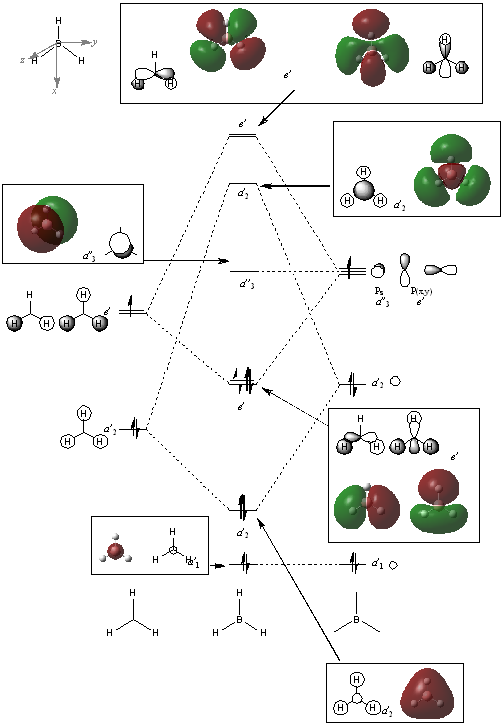
MO Diagram of BH3 reproduced with permission form Dr. Tricia Hunt's 2nd year Tutorial Problems [4].
Comparing the LCAO molecular orbitals (left) and the "real" molecular orbitals (right).
The LCAO molecular orbitals are merely approximations of the actual MOs. They give the general shape and symmetry of the molecular orbitals and also the relative size or contribution form each individual atomic orbital. We can also see where there could be possible nodes in the MOs. However, they do not show the actual shape formed after the atomic orbitals combined. The "real" MOs from computational methods show the shape after the combination instead of just merely overlapping of s and p orbitals. The qualitative MO theory in LCAO is not as accurate as computational methods, but they are still fairly accurate in terms of general shape and symmetry of the orbitals. It can still be considered rather useful as a first estimate to have a better sense of what the molecule should look like before using computational methods. In the computerised MOs, we can also see the effect of polarization of the MOs unlike in the LCAO MOs which have strictly fixed shapes which do not show polarization.
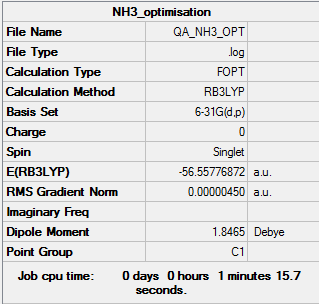
NH3
B3LYP/6-31G(d,p) level
Optimisation log file here
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
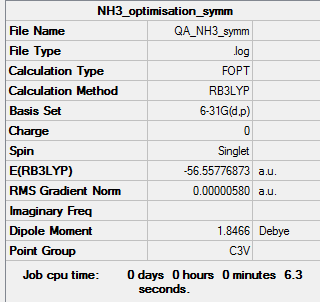
Symmetrised B3LYP/6-31G(d,p) level
Optimisation log file here
| Summary Data | Convergence |
|---|---|
 |

|
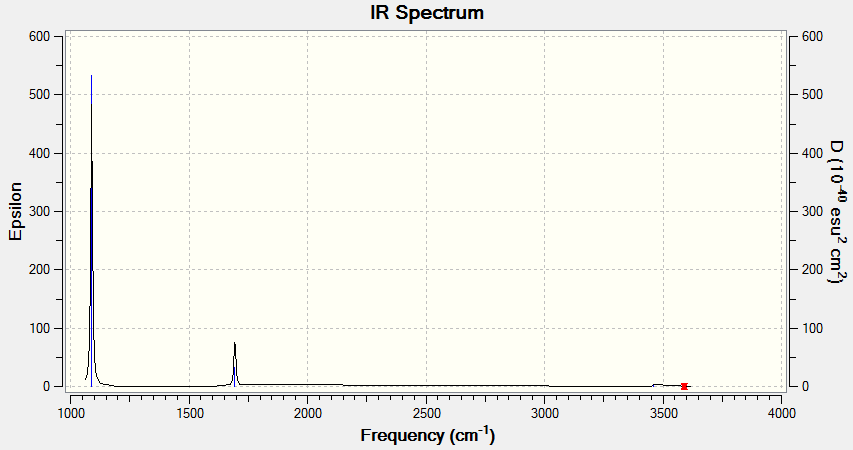
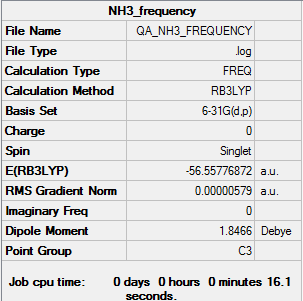
Vibrational Spectrum for NH3
Frequency log file here
| Summary Data | Low frequencies |
|---|---|
 |
| Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|
| 1089 | 145 | Yes | Bend |
| 1694 | 14 | Very slight | Bend |
| 1694 | 14 | Very slight | Bend |
| 3462 | 1 | No | Stretch |
| 3590 | 0 | No | Stretch |
| 3590 | 0 | No | Stretch |
NH3 MO Energies
Energy .fch file DOI:10042/90660
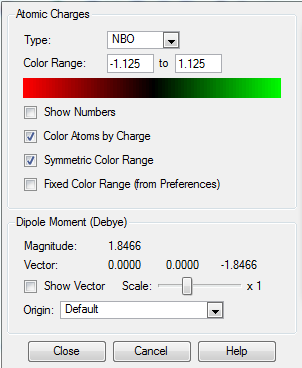
NBO Analysis of NH3
| Colour range | Image |
|---|---|
 |

|
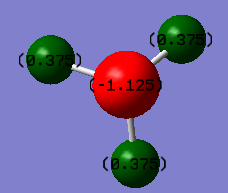
The charge numbers on individual atom in NH3 is shown in the image below:
| Hydrogen | Nitrogen |
|---|---|
| +0.375 | -1.125 |
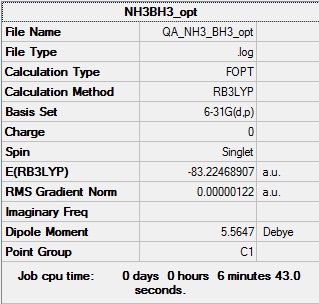
NH3BH3
B3LYP/6-31G(d,p) Optimisation
Optimisation log file here
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
B3LYP/6-31G(d,p) Symmetrisation
Symmetrisation log file DOI:10042/91192
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
Frequency of NH3bH3 B3LYP/6-31G(d,p)
Frequency log file DOI:10042/91241
| Summary Data | Low frequencies |
|---|---|
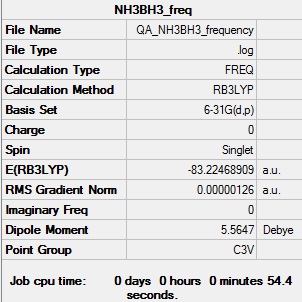 |
Vibrational Spectrum for NH3BH3
| No. | Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|---|
| 1 | 263 | 0 | No | Rocking |
| 2 | 633 | 14 | Very slight | Stretch |
| 3 | 633 | 4 | No | Twisting |
| 4 | 633 | 4 | No | Wagging |
| 5 | 1069 | 41 | Yes | Twisting |
| 6 | 1069 | 41 | Yes | Wagging |
| 7 | 1196 | 109 | Yes | Bend |
| 8 | 1204 | 3 | No | Bend |
| 9 | 1204 | 3 | No | Bend |
| 10 | 1329 | 114 | Yes | Bend |
| 11 | 1676 | 28 | Slight | Bend |
| 12 | 1676 | 28 | Slight | Bend |
| 13 | 2472 | 67 | Yes | Stretch |
| 14 | 2532 | 231 | Yes | Stretch |
| 15 | 2532 | 231 | Yes | Stretch |
| 16 | 3464 | 3 | No | Stretch |
| 17 | 3581 | 28 | Slight | Stretch |
| 18 | 3581 | 28 | Slight | Stretch |
Molecular Energies
| Molecule | Energy(a.u.) |
|---|---|
| NH3 | -56.5577687 a.u. |
| BH3 | -26.6153235 a.u. |
| NH3BH3 | -83.2246891 a.u. |
Calculating Bond Dissociation Energy
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
= -83.2246891 a.u. - [(-56.5577687 a.u.) + (-26.6153235 a.u.)] = -0.0515969 a.u. = -135.47 kJ/mol
The B-N bond in NH3BH3 can be considered to be a medium strength bond as its bond dissociation energy is relatively low compared to strong bonds like that of N≡N bond which has a bond dissociation energy of 945 kJ/mol as mentioned earlier. This B-N bond energy is also lower than the medium strength C-C bond (348 kJ/mol). However, it is still significantly higher than that of the weak hydrogen bond (approx. 3-32 kJ/mol). Therefore, the B-N bond can be considered as a medium strength bond. The low bond dissociation energy is expected of it as it is a dative bond involving the donation of a lone pair of 2p electrons form N into the vacant 2p orbital of B.
Lewis Acids Project section
Optimisation and Symmetrisation of 4 isomers of Al2Cl4Br2 using 631-G(d,p) and LANL2DZ pseudopotential
The four isomers formed from individual AlCl2Br monomers are given in the sub-sections below. 1. Terminal Br atoms are trans to each other 2. Terminal Br atoms are cis to each other 3. 1 bridging Br atom and 1 terminal Br atom 4. 2 bridging Br atoms, no terminal Br atom
trans-Br Isomer
Optimisation log file DOI:10042/98907
Table showing the optimisation of the trans isomer of Al2Cl4Br2
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
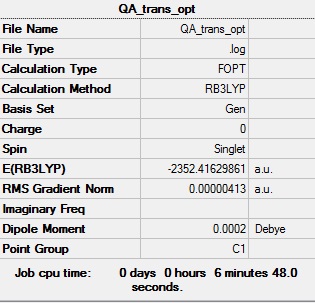 |
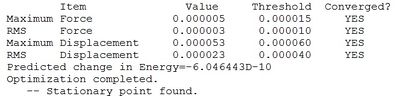 |
|
Symmetrisation log file DOI:10042/98921
Table showing the symmetrisation of the trans isomer Al2Cl4Br2
| Summary Data | Convergence |
|---|---|
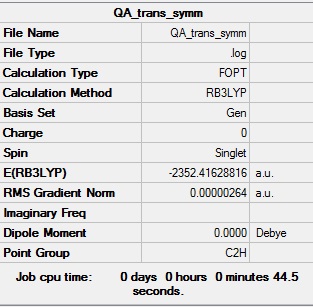 |

|
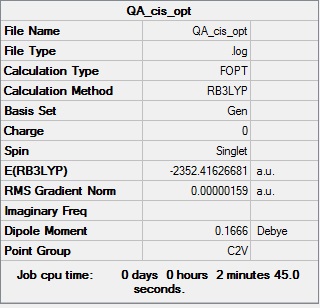
cis-Br Isomer
Optimisation log file DOI:10042/98914
Table showing the optimisation of the cis isomer of Al2Cl4Br2
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
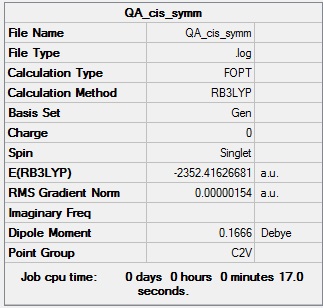
Symmetrisation log file DOI:10042/98934
Table showing the symmetrisation of the cis isomer of Al2Cl4Br2
| Summary Data | Convergence |
|---|---|
 |

|
1Bridging-Br Isomer
Optimisation log file DOI:10042/98978
Table showing the optimisation of the 1-Br bridge isomer of Al2Cl4Br2
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
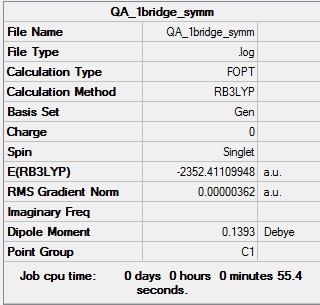
Symmetrisation log file DOI:10042/99037
Table showing the symmetrisation of the 1-Br bridge isomer of Al2Cl4Br2
| Summary Data | Convergence |
|---|---|
 |

|
2Bridging-Br Isomer
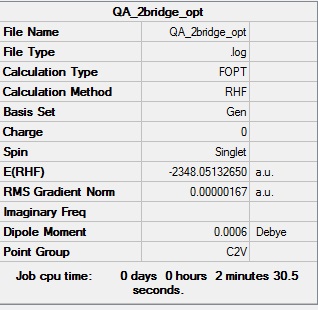
Optimisation log file DOI:10042/91726
Table showing the optimisation of the 2-Br bridge isomer of Al2Cl4Br2
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
Symmetrisation log file DOI:10042/91770
Table showing the symmetrisation of the 2-Br bridge isomer of Al2Cl4Br2
| Summary Data | Convergence |
|---|---|
 |

|
Comparison of energies of the isomers
| Isomers | Energy (a.u.) |
|---|---|
| trans | -2352.4162986 |
| cis | -2352.4162668 |
| 1-Br bridge | -2352.4110995 |
| 2-Br bridge | -2348.0513265 |
comparing the energies of isomers relative to the lowest energy isomer
The trans isomer was identified as the lowest energy isomer.
| Isomers | Relative Energy (a.u.) | Relative Energy kJ/mol |
|---|---|---|
| cis | 0.0000318 | 0.08 |
| 1-Br bridge | 0.0051991 | 13.65 |
| 2-Br bridge | 4.3649721 | 11460.24 |
discuss the position of the Br atoms with respect to the stability of the different conformers
Comparing the trans and cis isomers, the trans isomer is more energetically stable than the cis isomers. This is probably due to the steric factors because the trans isomer is less sterically strained due to the two large Br atoms on opposite ends of the molecule in a trans position. The cis isomer however experiences greater steric repulsion from the large 4p orbitals of the Br atoms being next to each other in the cis position.
The isomers with Br bridge is much more energetically unstable relative to the trans isomer as compared to the isomers with purely Cl bridging. The Al in AlCl3 has an empty 3p orbital while the Cl and Br atom have lone pair 3p and 4p electrons respectively. The Cl or Br donates a lone pair into the empty 3p orbital of the Al to form a bond hence forming a dimer with a Cl/Br bridge. The Cl bridge is more energetically stable because the 3p orbital of the Cl is closer in energy and size in relation to the empty 3p of Al, and hence have a better orbital overlap than the 4p orbital of Br which is larger and have a wide energy gap with empty 3p of Al. Therefore, the bond length of the bridging Al-Cl bond is shorter and stronger than that of the Al-Br bridge. Hence, the Br-bridging isomers are significantly energetically less stable than the trans isomer and even the less stable stable cis isomer. This effect is even more pronounced in the isomer in which both bridging atoms are Br instead of Cl.
AlCl2Br Monomer
Optimisation log file DOI:10042/101853
Table showing the optimisation of the trans isomer of Al2Cl4Br2
| Summary Data | Convergence | Jmol | |||
|---|---|---|---|---|---|
 |
 |
|
Bond Dissociation Energy of the lowest energy isomer
| Molecule | Energy (a.u.) |
|---|---|
| AlCl2Br | -1176.1901370 |
ΔE=E(Al2Cl4Br2)-[2 x E(AlCl2Br)]
= -2352.4162986 a.u. - [2 x (-1176.1901370 a.u.)] = -0.0360246 a.u. = -94.58 kJ/mol
is the product more or less stable than the isolated monomers?
The product is more stable than the isolated isomers. The ΔE from dimerisation is negative hence showing that dimerisation is more energetically favourable than existing as separate monomers. However, the dative bonds formed form the dimerisation are not very strong. They are relatively weak medium strength bonds since the bond dissociation energy is rather low (-94.58 kJ/mol for 2 Al-Cl dative bonds formed.) Hence, it is only slightly more enrgetically favourable and would probably exist as monomers at higher temperatures, similar to that in AlCl3.
Frequency Analysis
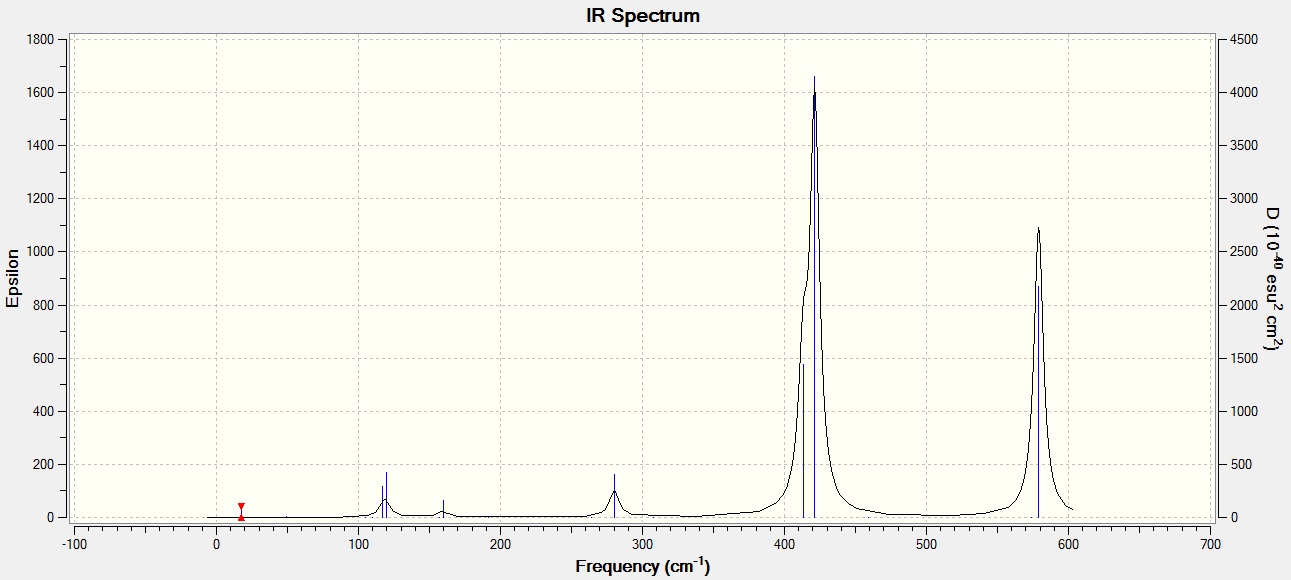
Frequencies of the trans isomer
Frequency log file DOI:10042/102436
| Summary Data | Low frequencies |
|---|---|
 |
| No. | Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|---|
| 1 | 18 | 0 | No | Bending |
| 2 | 49 | 0 | No | Twist |
| 3 | 73 | 0 | No | Bending |
| 4 | 105 | 0 | No | Bending |
| 5 | 110 | 0 | No | Rocking |
| 6 | 117 | 9 | very slight | Wagging |
| 7 | 120 | 13 | very slight | Bending |
| 8 | 157 | 0 | No | Rocking |
| 9 | 160 | 6 | Very slight | Wagging |
| 10 | 192 | 0 | No | Bend |
| 11 | 264 | 0 | No | Stretch |
| 12 | 280 | 29 | Slight | Stretch |
| 13 | 308 | 0 | No | Stretch |
| 14 | 413 | 149 | Yes | Stretch |
| 15 | 421 | 434 | Yes | Stretch |
| 16 | 459 | 0 | No | Stretch |
| 17 | 574 | 0 | No | Stretch |
| 18 | 579 | 316 | Yes | Stretch |
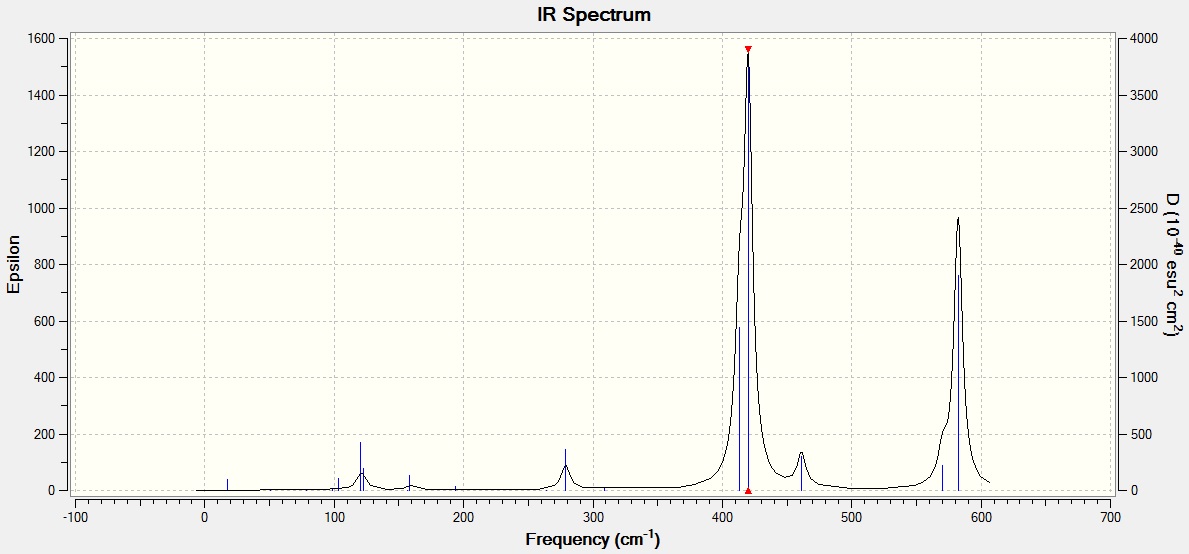
Frequencies of the cis isomer
Frequency log file DOI:10042/102255
| Summary Data | Low frequencies |
|---|---|
 |
| No. | Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|---|
| 1 | 17 | 0 | No | Bending |
| 2 | 51 | 0 | No | Twist |
| 3 | 79 | 0 | No | Bending |
| 4 | 99 | 0 | No | Bending |
| 5 | 103 | 3 | No | Twisting |
| 6 | 121 | 13 | very slight | Bending |
| 7 | 123 | 6 | No | Twist |
| 8 | 157 | 0 | No | Rocking |
| 9 | 158 | 5 | Very slight | Wagging |
| 10 | 194 | 2 | No | Bend |
| 11 | 264 | 0 | No | Stretch |
| 12 | 279 | 25 | Slight | Stretch |
| 13 | 309 | 2 | No | Stretch |
| 14 | 413 | 149 | Yes | Stretch |
| 15 | 420 | 411 | Yes | Stretch |
| 16 | 461 | 35 | Slight | Stretch |
| 17 | 570 | 32 | Slight | Stretch |
| 18 | 582 | 278 | Yes | Stretch |
Frequencies of the 1-Br bridge isomer
Frequency log file DOI:10042/102341
| Summary Data | Low frequencies |
|---|---|
 |
| No. | Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|---|
| 1 | 17 | 0 | No | Bending |
| 2 | 56 | 0 | No | Twist |
| 3 | 80 | 0 | No | Bending |
| 4 | 92 | 1 | No | Bending |
| 5 | 107 | 0 | No | Rocking |
| 6 | 110 | 5 | very slight | Wagging |
| 7 | 121 | 8 | very slight | Bend |
| 8 | 149 | 5 | very slight | Rocking |
| 9 | 154 | 6 | Very slight | Wagging |
| 10 | 186 | 1 | No | Bend |
| 11 | 211 | 21 | Slight | Stretch |
| 12 | 257 | 10 | Very slight | Stretch |
| 13 | 289 | 48 | Yes | Stretch |
| 14 | 384 | 153 | Yes | Stretch |
| 15 | 424 | 274 | Yes | Stretch |
| 16 | 493 | 107 | Yes | Stretch |
| 17 | 574 | 122 | Yes | Stretch |
| 18 | 615 | 197 | Yes | Stretch |
Frequencies of the 2-Br bridge isomer
Frequency log file DOI:10042/102397
| Summary Data | Low frequencies |
|---|---|
 |
| No. | Wavenumber | Intensity | IR Active? | Type |
|---|---|---|---|---|
| 1 | 17 | 0 | No | Bending |
| 2 | 68 | 0 | No | Twist |
| 3 | 93 | 0 | No | Rocking |
| 4 | 94 | 0 | No | Bending |
| 5 | 114 | 6 | Very slight | Wagging |
| 6 | 118 | 0 | No | Rocking |
| 7 | 134 | 12 | very slight | Bend |
| 8 | 146 | 0 | No | Rocking |
| 9 | 151 | 11 | Very slight | Wagging |
| 10 | 177 | 0 | No | Bend |
| 11 | 182 | 0 | No | Stretch |
| 12 | 254 | 139 | Yes | Stretch |
| 13 | 259 | 0 | No | Stretch |
| 14 | 347 | 228 | Yes | Stretch |
| 15 | 486 | 406 | Yes | Stretch |
| 16 | 515 | 0 | No | Stretch |
| 17 | 639 | 0 | No | Stretch |
| 18 | 649 | 423 | Yes | Stretch |
Discussion
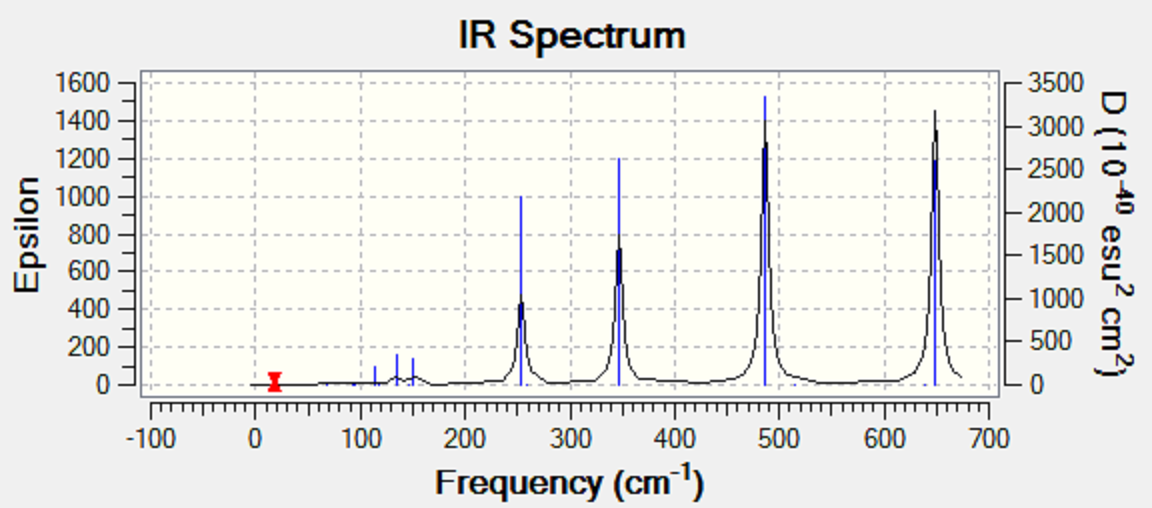
discuss the IR spectra with respect to the symmetry of each conformer, why do some spectra have more bands than others?
The trans isomer has 2 large obvious peaks at 579 cm-1 and another at 421 cm-1. However, the strong peak at 421 cm-1 is a combined peak with another fairly strong 413 cm-1. These 2 peaks are so close together that they appear to be one slightly distorted peak. These 2 strong signals represent the IR active stretches in the trans isomer. There are also 4 smaller peaks at 117 cm-1, 120 cm-1, 160 cm-1, and 280 cm-1, relating to bending modes. The peaks at 117 cm-1 and 120 cm-1 also appear as one small peak because they are very close in energy. The Al-Cl stretch at 579 cm-1 and the Al-Br stretch at 421 cm-1 were not close to experimentally obtained value in literature for the AlCl2Br monomer, which were given as 616 cm-1 and 560 cm-1 respectively [3]. This difference in values can be explained by the difference in symmetry with respect to the central Al atom. In the trans dimer, the molecule has a C2H symmetry and is tetrahedral with respect to the central Al atom where as the monomer AlCl2Br has a C2V symmetry and is trigonal planar instead. Hence, the vibrational energies associated with the Al-Cl and Al-Br stretches would vary between the monomer and the dimer. The differences in symmetry would result in a different change in net dipole moment for the same type of stretch.
The cis isomer has similar vibrational modes as the trans isomer with strong peaks representing stretching at 582 cm-1, 420 cm-1, and 413 cm-1, and 4 smaller peaks at 279 cm-1, 158 cm-1, 123 cm-1, and 121 cm-1. The peaks at 420 cm-1 and 413 cm-1, and the other two at 123 cm-1 and 121 cm-1 also appear as one large peak and one small peak respectively because they are very close in energy. The main difference between the cis isomer and the trans isomer is that the cis isomer has two additional fairly strong peaks at 570 cm-1 and 461 cm-1 whereas those peaks were not visible in the IR spectrum of the trans isomer. These two peaks appear in the cis isomer but not the trans isomer because of the relative positions of the Br atoms in each isomer. In the cis isomer, the stretching symmetric at 570 cm-1 and 461 cm-1 results in a net change in dipole moment since the two Br atoms are on the same side of the molecule whereas in the trans isomer, they are at opposite ends and any change in dipole moment due to stretching on one side is countered by an equal change in dipole moment at the other side in the opposite direction. Therefore, there is no net change in dipole moment in the trans isomer for the equivalent stretch as compared to the cis isomer, hence the stretch is IR inactive [2]. Thus, the IR spectrum of the cis isomer has more peaks than that in the trans isomer.
The 1-Br bridge isomer have many IR active peaks visible in the IR spectrum as compared to the other three isomers. This is because the 1-Br bridge isomer is highly asymmetrical as compared to the others, with a symmetry of C1. There is only 1 terminal Br atom and 3 terminal Cl atom. Also, the dimer consists of 1 Br bridge and 1 Cl bridge. This collectively breaks the symmetry of the molecule, leading to more vibrational modes to cause a net change in dipole moment, which otherwise would not in a more symmetric isomer. Hence, more peaks appear, and the vibrational energies also differ in magnitude due to the addition of an Al-Br bridge stretching and the asymmetric terminal Cl-Al-Cl stretch.
The 2-Br bridge isomer however, have less peaks than the 1-Br bridge isomer because the symmetry of the molecule is restored with 2 bridgu=ing Br atoms and 4 terminal Cl atoms. The 2-Br bridge isomer has a symmetry of D2H. The main difference is that it has some peaks that the trans isomer do not, which is the 649 cm-1 peak which corresponds to the asymmetric stretch of terminal Al-Cl bonds and also symmetric and asymmetric stretching of the Al-Br bridges (486 cm-1 and 347 cm-1) respectively.
an advantage of computational chemistry is that you can calculate the position of bands that are not experimentally active, what is the requirement for an IR band to be active?
For the vibrational modes of a molecule to be IR active or be seen on the IR spectrum, that particular vibrational mode must first result in a change in the net dipole moment of the molecule [2]. As such, symmetric stretching of symmetric molecules are not detected by IR spectroscopy. Therefore, some of the isomers produce more peaks in the IR spectra than other isomers despite all of them having the same number of 18 vibrational modes.
discuss the position and nature of Al-Br stretching vibrations with respect to the terminal or bridging position of the Br atom. Placing images of the spectra side by side is not sufficient, tabulate the key vibrational frequencies (remember that they can reorder, so be sure you are comparing similar modes). The key word here is "discuss" just presenting images and tables is not sufficient, you must interpret your results.
| Type | Bridging Al-Br | Terminal Al-Br |
|---|---|---|
| Symmetric synchronised | 259 cm-1 | 459 cm-1 |
| Symmetric alternating | 347 cm-1 | 421 cm-1 |
| Asymmetric synchronised | 254 cm-1 | 574 cm-1 |
| Asymmetric alternating | 182 cm-1 | 579 cm-1 |
In general, the stretching frequencies of the terminal Al-Br bond are of higher energy than that of the bridging Al-Br bonds. This means that the terminal Al-Br bonds are significantly stronger than the bridging Al-Br bond. This is explained by the direct correlation between energy and the frequency of vibration in terms of wavenumber as given by the Planck-Einstein relation:
Where v is the frequency in wavenumbers and c is the speed of light.
From the above equation, a higher magnitude of vibrational frequency would equate to higher energy, therefore the bond energy of terminal Al-Br is higher than that of the bridging Al-Br. This is to be expected because the bridging bond is a dative bond from the donation of a lone pair of 4p electrons form Br into the vacant 3p orbital of Al, whereas the terminal bond is a strong bond formed by the orbital overlap and pairing of a single 4p electron from Br and a single 3p electron from Al.
MO Energies
MO fch. file DOI:10042/105221
5 non-core orbitals
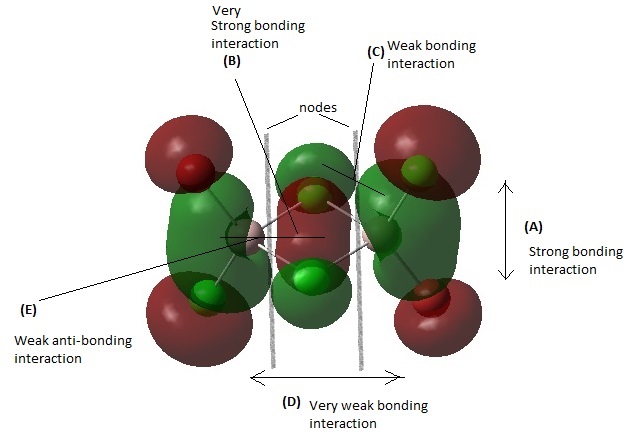
MO 41
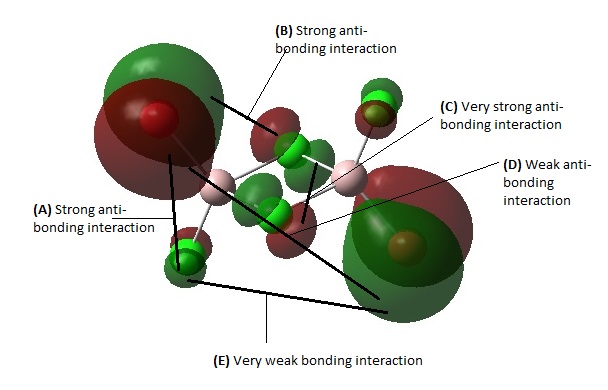
Overall, this MO is highly bonding due to many bonding interactions, weak anti-bonding character, and presence of few nodes. There is a very strong bonding interaction in the form of head on orbital overlap between the 2 bridging Cl atoms at (B). There are also some very strong bonding interactions between the terminal halogens at the Cl-Al-Br bonds at (A) with good orbital overlap. At (C) there is some weak bonding interaction through space between the bridging Cl atom and the terminal Cl/Br atom. At (D) there is some very weak bonding interaction through space between the terminal halogens. At (E) there is a weak anti-bonding interaction between the bridging Cl atoms and the Al center. There are 2 nodal planes between Each side of the Al-Cl bridge.
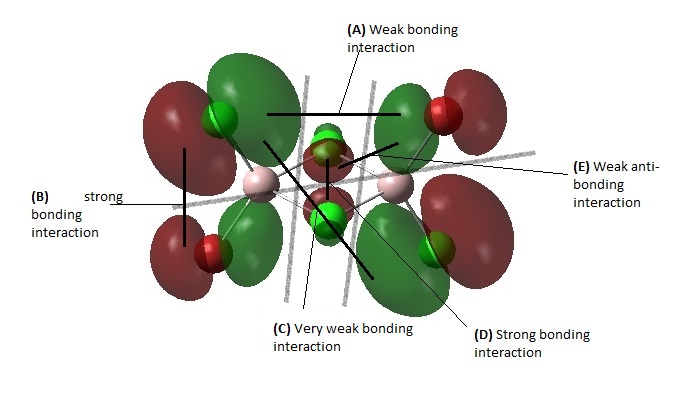
Overall, the MO is moderately bonding due to many bonding interactions and weak anti-bonding character, with 3 nodal planes. At (B), there is strong bonding interaction through the terminal Cl-Al-Br. At (A) there's some weak bonding interaction through space between the terminal Cl and terminal Br. At (C), there is some weak bonding interaction between the trans terminal Cl atoms. Similarly, there is also some weak bonding interaction between the trans terminal Br atoms. There is also a strong bonding interaction through head-on overlap between the two bridging Cl atoms at (D). There is only 1 weak anti-bonding interaction through space between the bridging Cl and the terminal Cl/Br. There are 2 nodal planes between Each side of the Al-Cl bridge and a third nodal plane that cuts horizontally across the molecule between the terminal halogens, along the Al center.
MO 46
This MO is intermediate in terms of bonding and anti-bonding character. It has fairly strong bonding interactions between the two bridging Cl atoms at (D) and weak through space bonding interaction between the terminal halogens on the same side at (A). It has a fairly strong anti-bonding interaction between the terminal Br and terminal Cl on the same Al atom at (B) and also a weak anti-bonding interaction at (C) between the bridging Cl and terminal Br/Cl. There are 2 nodal planes between Each side of the Al-Cl bridge and a third nodal plane that cuts horizontally across the molecule between the terminal halogens, along the Al center.
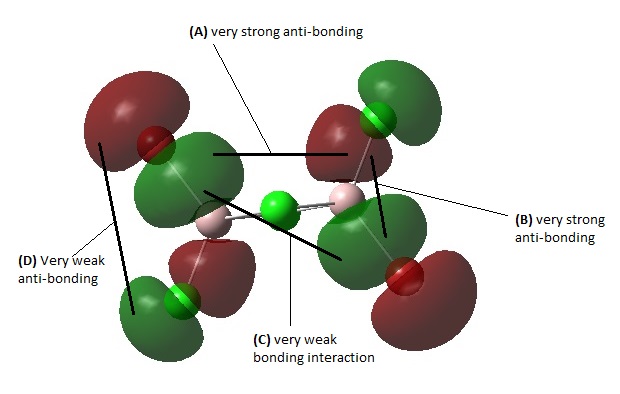
MO 45
This is a moderately anti-bonding MO due to it having more strong anti-bonding interactions overall than bonding interactions. Firstly, there is some very strong anti-bonding interaction between the two out-of-phase AOs of the terminal Br and terminal Cl at (B). There is also a strong anti-bonding interaction through space between terminal Cl and the terminal Br on the opposite Al atom as shown in (A).There is some very weak bonding interaction between terminal halogens trans to each other at (C). There is also no AO interactions contributed form the bridging Cl atoms, leading to an overall anti-bonding MO. There is a nodal plane that cuts horizontally across the molecule between the terminal halogens, along the Al center, and also a large non-bonding region at the Cl-bridge.
MO 52
This is a highly anti-bonding MO due to it having much more strong anti-bonding interactions and fewer bonding interactions overall. At (A) there is a very strong anti-bonding interaction along the terminal Cl-Al-Br bond between out-of-phase p AOs of Cl and Br. There is also Very strong anti-bonding interaction between the the two bridging Cl atoms due to sideways out-of-phase overlap at (C). At (B), there is a fairly strong anti-bonding interaction through space between the bridging Cl and the terminal Br/Cl atom. At (D) there is a very weak anti-bonding interaction between the two Br/Cl atoms trans to each other across the Cl bridge. There is a very weak bonding interaction through space between the terminal Cl on one Al atom and the terminal Br on the other Al atom as shown in (E). There are 2 nodal planes between Each side of the Al-Cl bridge. A third nodal plane cuts horizontally across the molecule between the terminal halogens, along the Al center. A fourth nodal plane cuts vertically in the plane of the Cl-Al-Br terminals.
References
1. Johnson, D. Arthur, ed. Metals and chemical change. Vol. 1. Royal Society of Chemistry, 2002.
2. Atkins, P. ; de Paula, J. ; Elements of physical chemistry (5th ed. ed.). 2009 p. 459.
3. Himmel, H. ; Bahlo, J. ; Haussmann, M. ; Kurth, F. ; Stoesser, G. ; Schnoeckel, H. ; Inorganic Chemistry, 2002 , vol. 41, # 19 p. 4952 - 4960
4. Hunt, P.; Tutorial Problems, 2nd Year lecture course on Molecular Orbitals, http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year3/Tut_MO_diagram_BH3.pdf
5. Atkins, P. ; de Paula, J. ; Physical Chemistry, 9th ed., 2010, p.932
6. Dougherty, R. C. "Temperature and pressure dependence of hydrogen bond strength: A perturbation molecular orbital approach." The Journal of chemical physics 109.17 (1998): 7372-7378.
7. Atkins, P. ; de Paula, J. ; Physical Chemistry, 9th ed., 2010, p.372-378