Rep:Mod:angela910118
Introduction
Major purpose of this experiment
The molecular mechanics method is good for finding the structure of the product but not suitable for analysis the structure of the transition state, in other words it is not helpful for describing the formation or cleavage of the bonds.By using molecular orbital-based methods, the potential surface of the reaction is analysed in order to determined the location and structure of the transition state. Information like activation energy, the reaction energy path can be calculated out.In this case, we focus on Diels Alder cycloaddition reactions as well as cope rearrangement
What programme is used
GaussView 5.0.9 is used here.
Cope Rearrangement
In this section, different type of geometries of1,5-hexadiene is analysed below. In total, there are 10 type of structures but we will only discuss 4 of them.
Optimizing the Reactants and Products
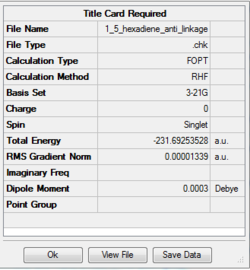
Optimizing anti (2) 1,5-hexadiene
Job type: Optimisation Method: Hartree Fock Basis set: 3-21G
The D space calculation is provided:[| click here]
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.001291 0.001800 YES
RMS Displacement 0.000349 0.001200 YES
Predicted change in Energy=-2.285645D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is Ci
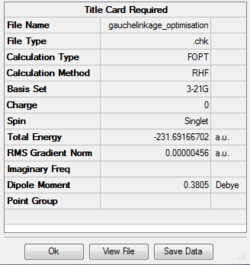
Optimizing gauche (2) 1,5-hexadiene
Job type: Optimisation Method: Hartree Fock Basis set: 3-21G
The D space calculation is provided:[| click here]
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000007 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000418 0.001800 YES
RMS Displacement 0.000139 0.001200 YES
Predicted change in Energy=-2.077361D-09
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C2
Optimizing anti (1) 1,5-hexadiene
Job type: Optimisation Method: Hartree Fock Basis set: 3-21G
The D space calculation is provided:[| click here]
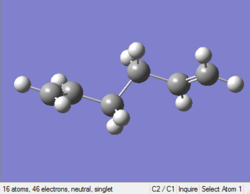
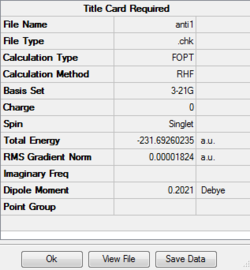
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000056 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.001583 0.001800 YES
RMS Displacement 0.000459 0.001200 YES
Predicted change in Energy=-2.090750D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C2
Optimizing gauche (3) 1,5-hexadiene
Job type: Optimisation Method: Hartree Fock Basis set: 3-21G
The D space calculation is provided:[| click here]
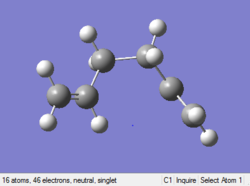
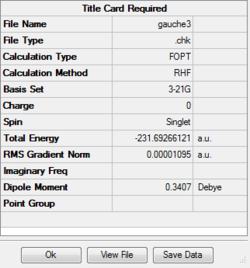
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000033 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.001259 0.001800 YES
RMS Displacement 0.000424 0.001200 YES
Predicted change in Energy=-1.726756D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C1
Comparison bewtween anti and gauche 1,5-hexadiene
All four 1,5 hexadiene calcualtion is carried out under this condition: Job type: Optimisation Method: Hartree Fock Basis set: 3-21G
| Molecule Conformation | Final Energy/ a.u. | RMS Gradient Norm /a.u. | Dipole moment /Debye | Point Group |
|---|---|---|---|---|
| anti(2) | -231.69254 | 0.00001339 | 0.0003 | Ci |
| anti(1) | -231.69260 | 0.00000456 | 0.2021 | C2 |
| gauche(2) | -231.69167 | 0.00001824 | 0.3805 | C2 |
| gauche(3) | -231.69266 | 0.00001095 | 0.3407 | C1 |
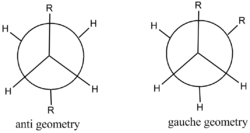
According to the table and graph above,the final energy,electronic energy of two anti conformations are more negative than that of two gauche geometry. As the two substituents on the gauche is next to each other(the angle between two subsitituents is 60),the steric effect makes the structure to be more unstable, which gauche geometry results in having higher final energy.However, the two substituents are 180 degree away from each other.According to the Appendix 1, the relative energy of gauche 3 is 0 kcal/mol while that of gauche 2 is 0.62 kcal/mol. The difference of relative energy between two anti geometries is small,anti(2) 0.4 kcal/mol, anti (1) 0.8 kcal/mol.
Further Optimizing anti (2) 1,5-hexadiene
Job type: Optimisation Method: DFT B3LYP Basis set: 6-31G(d)
The D space calculation is provided:[| click here]
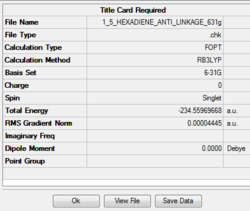
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000075 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000581 0.001800 YES
RMS Displacement 0.000228 0.001200 YES
Predicted change in Energy=-8.253337D-08
Optimization completed.
-- Stationary point found.
The point group of this structure after symmetric is Ci,which is exactly the same as the point group gained by calculation using basis set 3-21G.In other words, the structure of the moleucle dose not have sinificant change after being optimized at two different level.
For method of 3-21G, the final energy of the molecule is -231.69 a.u.For method of 6-31G(d), the final energy of the molecule is -231.56 a.u. which is relatively lower.
However, although the point group of the two optimized molecules dose not change, the individual bond lengths have been changed. The carbon carbon double bonds are expected to shorter in the calculation using basis set 3-21G (3-21G, 1.316 6-31G(d),1.338)and the carbon single bonds are also expected to be shorter in the calculation using basis set 3-21G (3-21G, 1.553 6-31G(d), 1.555). However the dihedral angles for both optimizations are about the same, around 180 degree.
Frequncy anaylsis anti (2) 1,5-hexadiene
Two different basis sets are used for frequency calculations.
First calcualtion:
Job type: Frequency Method: Hartree Fock Basis set: 3-21G
The D space calculation is provided:[| click here]
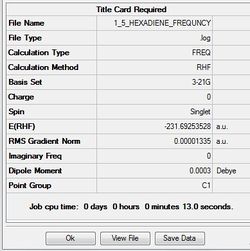
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.001680 0.001800 YES
RMS Displacement 0.000416 0.001200 YES
Predicted change in Energy=-1.964588D-08
Optimization completed.
-- Stationary point found.
Second calcualtion:
Job type: Frequency Method: DFT B3LYP Basis set: 6-31G(d)
The D space calculation is provided:[| click here]
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000107 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.000529 0.001800 YES
RMS Displacement 0.000238 0.001200 YES
Predicted change in Energy=-8.713057D-08
Optimization completed.
-- Stationary point found.
Thermochemistry
Four quantities has been recorded in the log output file,which have 4 different meanings in turns of energy:
(1)Sum of electronic and zero-point Energies represents the sum of the potential energy at 0 K and zero potential energy.E=Eelec+ZPE
(2)Sum of electronic and thermal Energies represents energy at 298.15 K and 1 atm of pressure which includes the contribution of translation, vibration, rotation.E=E+Evib+Erot+Etrans
(3)Sum of electronic and thermal Enthalpies represents the energy associated with RT. H=E+RT
(4)Sum of electronic and thermal Free Energies represents the entropic contribution of free energy. G=H-TS
Data gained from calculation using basis set 3-21G
Sum of electronic and zero-point Energies= -231.539540 Sum of electronic and thermal Energies= -231.532566 Sum of electronic and thermal Enthalpies= -231.531622 Sum of electronic and thermal Free Energies= -231.570914
Data gained from calculation using basis set 6-31G(d)
Sum of electronic and zero-point Energies= -234.416252 Sum of electronic and thermal Energies= -234.408952 Sum of electronic and thermal Enthalpies= -234.408008 Sum of electronic and thermal Free Energies= -234.447896
Comparison between experimental data and the literature data
| Energy | Data gained from calcualtion using 3-21G/ a.u. | literature value for calcualtion using 3-21G/ a.u. | Data gained from calcualtion using 6-31G(d)/ a.u. | literature value for calcualtion using 6-31G(d)/ a.u. |
|---|---|---|---|---|
| E=Eelec+ZPE | -231.539540 | -231.539539 | -234.416252 | -234.469203 |
| E=E+Evib+Erot+Etrans | -231.532566 | -231.532566 | -234.408952 | -234.461856 |
| H=E+RT | -231.531622 | -234.408008 | ||
| G=H-TS | -231.570914 | -234.447896 | ||
| Electronic energy | -231.69253528 | -231.692535 | -234.55969668 | -234.611710 |
All the experiment data is almost the same as the literature value. This indicateds the calculation preformed is correct.
The predicted IR spectrum
First calcualtion:
Job type: Frequency Method: Hartree Fock Basis set: 3-21G
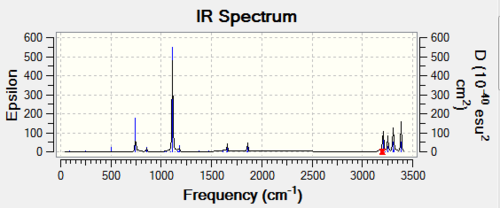
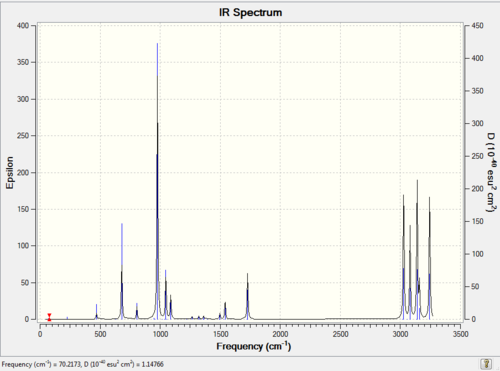
Second calcualtion:
Job type: Frequency Method: DFT B3LYP Basis set: 6-31G(d)
Optimizing Chair and Boat Transition Structures
As the chair and boat transition structures are both made from allyl fragments.In this section, optimisation calculation is carried out for allyl fragments, chair transition, boat transition.Frequency analysis and IRC calculations are also preformed in order to discover the physical properties of the transition states
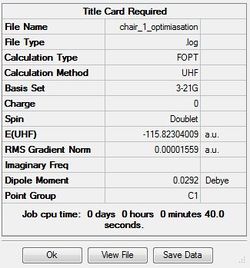
Optimizing allyl fragment
Job type: Optimization Method: Hartree Fock Basis set: 3-21G
The log file of optimization:click here
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000045 0.000450 YES
RMS Force 0.000021 0.000300 YES
Maximum Displacement 0.001475 0.001800 YES
RMS Displacement 0.000382 0.001200 YES
Predicted change in Energy=-2.238101D-07
Optimization completed.
-- Stationary point found.
The point group of this stucture is C1
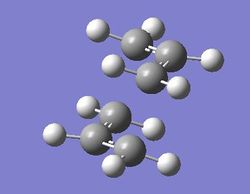
Optimizing chair transition state
There are two ways to optimize this transition state.The easiest way is using hessian method to calculate.In order to do so, the estimated transition structure has to be reasonable.Otherwise,a better calculation is preformed by freezing the reaction coordinate.Two different ways of calculation is preformed below.
Optimization by hessian method
Job type: Opt+Fre optimization to a TS(Berny) Method: Hartree Fock Basis set: 3-21G Additional keyword is Opt=NoEigen
The distance of two pairs of carbon which will form two carbon carbon single bonds is set to 2.2 Å that is a bond length of a carbon carbon single bond.
The log file of optimisation:click here

The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000385 0.001800 YES
RMS Displacement 0.000073 0.001200 YES
Predicted change in Energy=-1.460758D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture is C1
The thermochemistry of the chair transition state
Sum of electronic and zero-point Energies= -231.466700 Sum of electronic and thermal Energies= -231.461341 Sum of electronic and thermal Enthalpies= -231.460397 Sum of electronic and thermal Free Energies= -231.495206
According to the frequency calculation, the data gained indicates there is only one imaginary frequency which is -818cm-1
Low frequencies --- -817.9850 -0.9874 -0.0007 -0.0006 0.0006 1.5634 Low frequencies --- 2.9830 209.5556 396.0027
Optimization by frozen coordinate method
Step One
Before preforming the optimization calculation,freeze two pairs of carbon atoms which will form new sigma bond though the the Redundant Coord Editor in the edit menu.
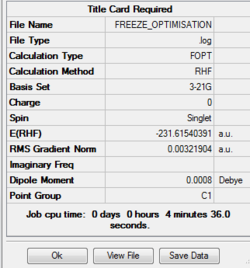
Job type: Optimization,optimize to a TS(Berny) Method: Hartree Fock Basis set: 3-21G Additional keyword is Opt=NoEigen
The distance of two pairs of carbon which will form two carbon carbon single bonds is set to 2.2 Å that is a bond length of a carbon carbon single bond.
The log file of optimisation:click here
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000891 0.001800 YES
RMS Displacement 0.000124 0.001200 YES
Predicted change in Energy=-2.748211D-08
Optimization completed.
-- Stationary point found.
The point group of this structure is C1
Step Two
Before preforming the optimization calculation,change "Bond" and "Freeze Coordinate" to "Bond" and "Derivative" in the Redundant Coord Editor of the edit menu.
Job type: Opt+Fre optimization to a TS(Berny) Method: Hartree Fock Basis set: 3-21G Additional keyword is Opt=NoEigen
Because of step one. the breaking or forming carbon carbon sigma bond is fixed to 2.2 Å.
The log file of optimization:click here
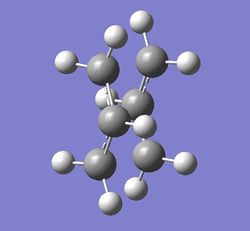
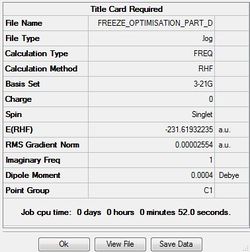
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000012 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.001302 0.001800 YES
RMS Displacement 0.000231 0.001200 YES
Predicted change in Energy=-1.296662D-07
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C1
The thermochemistry of the chair transition state
Sum of electronic and zero-point Energies= -231.466701 Sum of electronic and thermal Energies= -231.461341 Sum of electronic and thermal Enthalpies= -231.460397 Sum of electronic and thermal Free Energies= -231.495207
Comparison of two optimization method
From the two optimized transition structure, the point groups of two optimized transition states are the same, C1. Moreover, the distances between two allyl fragments are the same,2.02 Å.The final energy of the transition state is lower in hessian method,-231.61932246 a.u.(final energy of frozen coordinate method is -231.61540391 a.u. ).
Optimizing boat transition state
Compared to the method used to calculate the chair transition state, the method used for boat transition state is QST2. The reason for using QST2 method is that is possibly to specify the reactant and product of the reaction and is possible to discover the transition state of the reaction.In order to carry out OST2 calculation, the label of the atoms of reactant molecule is changed manually, which therefore it correspond to the label of the atoms of product molecule.
Failed Calculation
Job type: Opt+Freq, optimize to TS (QST2) Method: Hartree Fock Basis set: 3-21G
The log file of optimization:click here
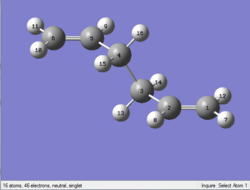
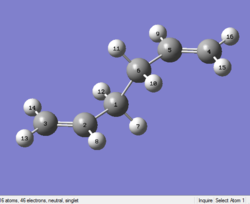

The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000045 0.000450 YES
RMS Force 0.000021 0.000300 YES
Maximum Displacement 0.001475 0.001800 YES
RMS Displacement 0.000382 0.001200 YES
Predicted change in Energy=-2.238101D-07
Optimization completed.
-- Stationary point found.
The point group of this structure is C2H
The thermochemistry of the chair transition state
Sum of electronic and zero-point Energies= -231.466701 Sum of electronic and thermal Energies= -231.461341 Sum of electronic and thermal Enthalpies= -231.460397 Sum of electronic and thermal Free Energies= -231.494552
As you can see above, the optimized structure is similar to a chair transition state. What is expected is a boat transition state. So the calculation is failed.
Successful Calculation
In order to obtain the correct transition state, a few changes in the input file of the calculation is made. The dihedral angle is changed to 0 degree and the C-C-C bond is altered to 100 degree.
Job type: Opt+Freq, optimize to TS (QST2) Method: Hartree Fock Basis set: 3-21G
The log file of optimization:click here


The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000081 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.001318 0.001800 YES
RMS Displacement 0.000411 0.001200 YES
Predicted change in Energy=-7.023936D-07
Optimization completed.
-- Stationary point found.
The point group of this structure is C2V
The thermochemistry of the chair transition state
Sum of electronic and zero-point Energies= -231.450931 Sum of electronic and thermal Energies= -231.445302 Sum of electronic and thermal Enthalpies= -231.444358 Sum of electronic and thermal Free Energies= -231.479776
According to the frequency calculation, the data gained indicates there is only one imaginary frequency which is -839cm-1
Low frequencies --- -839.3504 -5.5667 -0.0016 -0.0013 -0.0010 2.3760 Low frequencies --- 4.3926 155.4092 381.8052
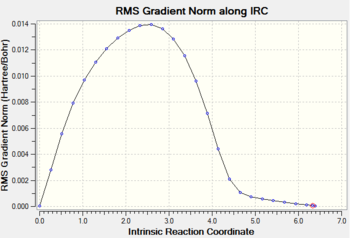
Intrinsic Reaction Coordinate calculation of chair transition state
It is impossible for us to predict which conformer will be formed from the transition state though the reaction paths. Intrinsic Reaction Coordinate calculation helps us to follow the minimum energy reaction path way from the transition state to a local potential minimum on the surface.
First Intrinsic Reaction Coordinate calculation
Job type: IRC, forward direction, force constant once, calculate always, the number of points along the IRC= 50 Method: Hartree Fock Basis set: 3-21G

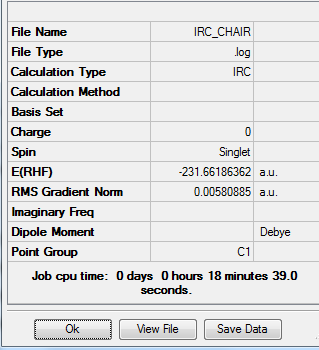
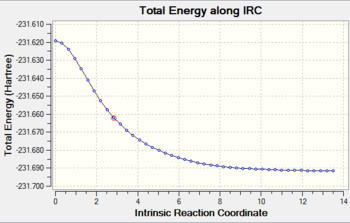
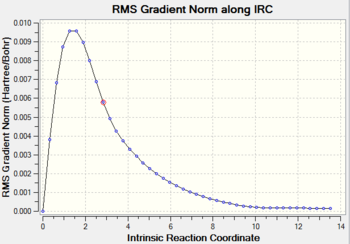
As you can see from the graph above, the RMS gradient is almost zero. It incicates that the calculation has almost find the local minimun of the potential surface.
Optimisation after Intrinsic Reaction Coordinate calculation
Sometimes the IRC calculation will not reach a minimum geometry. Another optimization is preformed for the last point of the IRC calculation. In this case,although the data of IRC indicates the minimum geometry is almost reached, the optimization is still preformed to double check whether the data gained above is correct or not.
Job type: Optimization Method: Hartree Fock Basis set: 3-21G
The log file of optimization:click here
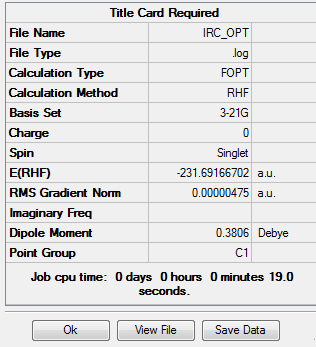
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000299 0.001800 YES
RMS Displacement 0.000091 0.001200 YES
Predicted change in Energy=-2.412878D-09
Optimization completed.
-- Stationary point found.
The point group of this structure is C1
As you can see the final energy of the optimization is slight lower than the energy calculated by IRC method.It proves that the IRC calculation reaches the minimum geometry.
Activation energy analysis
In this section, both chair and boat transition state is optimized at a more accurate level,B3LYP/6-31G (d) level. The following frequency analysis helps us to obtain the thermochemistry data in order to calculate the activation energy of both transition states
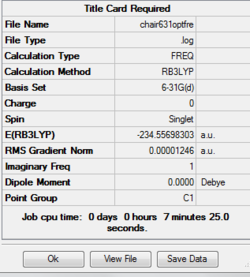
Reoptimizing chair transition state
Job type: Opt+Fre optimization to a TS(Berny) Method: B3LYP Basis set: 6-31G(d) Additional keyword is Opt=NoEigen
The log file of optimization:click here
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000027 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000108 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.291256D-09
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C1
The thermochemistry of the chair transition state
Sum of electronic and zero-point Energies= -234.414929 Sum of electronic and thermal Energies= -234.409008 Sum of electronic and thermal Enthalpies= -234.408064 Sum of electronic and thermal Free Energies= -234.443814
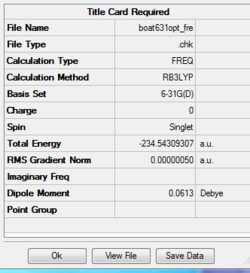
Reoptimizing boat transition state
Job type: Opt+Fre optimization to a TS(Berny) Method: B3LYP Basis set: 6-31G(d) Additional keyword is Opt=NoEigen
The log file of optimization:click here
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000018 0.001800 YES
RMS Displacement 0.000004 0.001200 YES
Predicted change in Energy=-1.210786D-11
Optimization completed.
-- Stationary point found.
The point group of this structure is C1
The thermochemistry of the boat transition state
Sum of electronic and zero-point Energies= -234.402342 Sum of electronic and thermal Energies= -234.396008 Sum of electronic and thermal Enthalpies= -234.395063 Sum of electronic and thermal Free Energies= -234.431753
Discussion
| Energy | Chair conformation | Boat conformation | anti(2) 1,5-hexadiene |
|---|---|---|---|
| E=Eelec+ZPE | -234.414929 | -234.402342 | -234.469203 |
| E=E+Evib+Erot+Etrans | -234.409008 | -234.396008 | -234.461856 |
| H=E+RT | -234.408064 | -234.395063 | |
| G=H-TS | -234.443814 | -234.431753 | |
| Electronic energy | -234.55698303 | -234.54309307 |
| Activation energy | Chair conformation | Boat conformation |
|---|---|---|
| Activation energy at 0 K experiment data | 34.1 | 42 |
| Activation energy at 0 K literature data | 33.5 ± 0.5 | 44.7 ± 2.0 |
1 a.u. = 627.509 kcal/mol
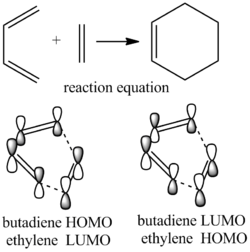
The Diels Alder Cycloaddition
The Diels Alder reaction is one type of the pericyclic reactions. The most important feature is that the pi bonds of the dieneophile and diene will overlap with each other to form a new sigma bond.In terms of molecular orbitals, this new sigma bond will only formed under these condition:the HOMO of one reactant interacts with the other reactant's LUMO; the overlap between two reactant orbital is significant enough;the overlap orbitals are having the same symmetry. In this case, the HOMO and LUMO of the butadiene are both symmetric and the HOMO and LUMO of ethyleneare both anti symmetric. The new formed sigma bond therefore is anti symmetric.
Optimisation the reactant
Butadiene and ethylene are both optimised. Each HOMO and LUMO are predicted graphically.
Butadiene
Job type: Optimisation Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
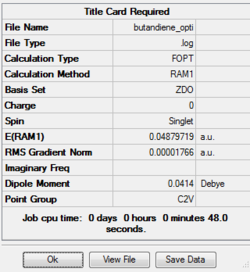
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000046 0.000450 YES
RMS Force 0.000016 0.000300 YES
Maximum Displacement 0.000237 0.001800 YES
RMS Displacement 0.000111 0.001200 YES
Predicted change in Energy=-1.212207D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C2V
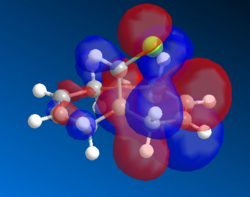
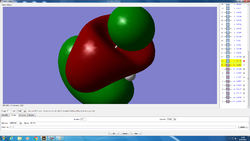
HOMO is anti-symmetric.LUMO is symmetric
Ethylene
Job type: Optimisation Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000162 0.000450 YES
RMS Force 0.000049 0.000300 YES
Maximum Displacement 0.000414 0.001800 YES
RMS Displacement 0.000220 0.001200 YES
Predicted change in Energy=-3.787280D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C2V
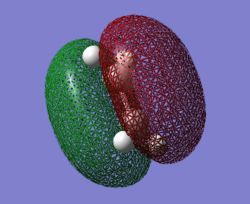
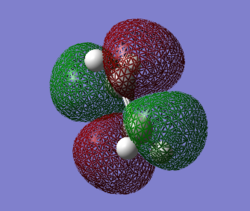
HOMO is symmetric.LUMO is anti-symmetric
Optimisation the transition state
Optimisation and frequncy anaylsis
Job type: Opt+Freq Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
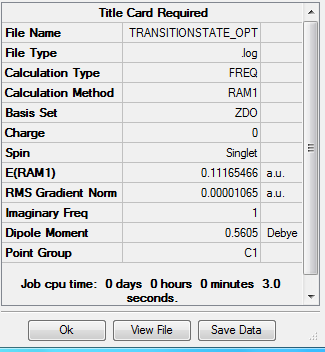
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000741 0.001800 YES
RMS Displacement 0.000155 0.001200 YES
Predicted change in Energy=-1.689969D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C1
HOMO and LUMO
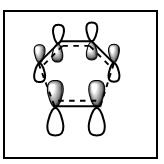
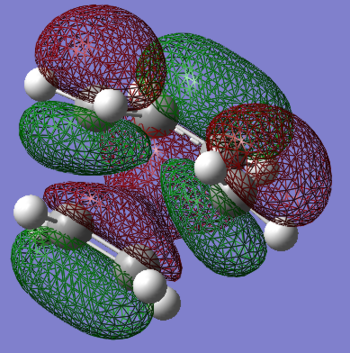
HOMO is anti-symmetric.LUMO is symmetric
The partially formed canrbon carbon bond is 2.30 Å. Typical sp3 carbon bond is 1.54 Å. Typical sp2 carbon bond is 1.46 Å. The van der Waals radius of the carbon is 1.7 Å. It indicates the the carbon carbon bond is not fully bonded and it is still in transition state.
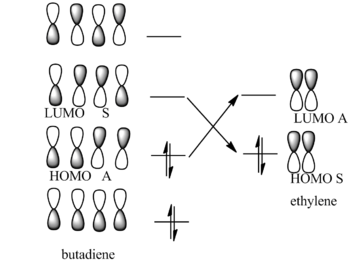
In order to interact, two molecular orbitals should have the same symmetry and similar energy level.So the transition state HOMO and LUMO is the results of the butadiene and ethylene HOMO LUMO interation.
Predicted IR spectrum
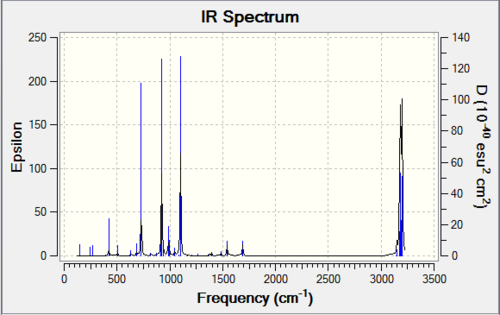
Imaginary frequency and Lowest frequency
The imaginary frequency is -956.09 cm-1.The vibration is perpendicular to the node planer and it is related to the formation of the new carbon bond. So it is bonds synchronous
The lowest frequency is 147.33 cm-1.The vibration is along the node planer and it is not related to the formation of the new carbon bond. So it is bonds asynchronous.
IRC anaylsis
Job type: IRC, both directions, force constant once, calculate always, the number of points along the IRC= 50 Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
Gradient and energy change along the IRC claculation
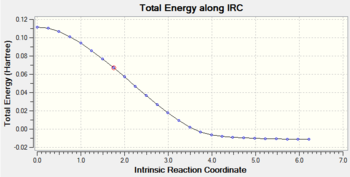
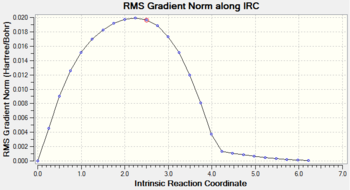
The RMS gradient along the IRC calculation is close to zero, which indicates that the local minimum is located.
The regioselectivity of the Diels Alder Reaction
Exo transition state
Optimisation and frequncy anaylsis
Job type: Opt+Freq Method: semi-empirical Basis set: AM1
The log file of optimisation:click here as the log file is too big to upload, it is saved as a work file.
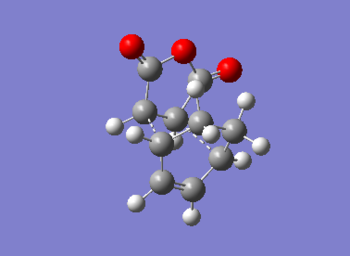
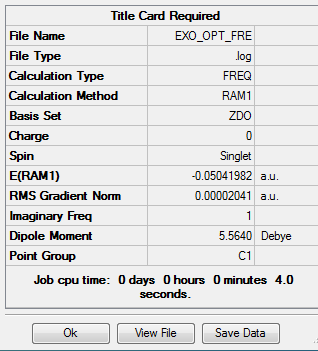
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000070 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.000755 0.001800 YES
RMS Displacement 0.000198 0.001200 YES
Predicted change in Energy=-3.582496D-08
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C1
HOMO and LUMO

Predicted IR spectrum
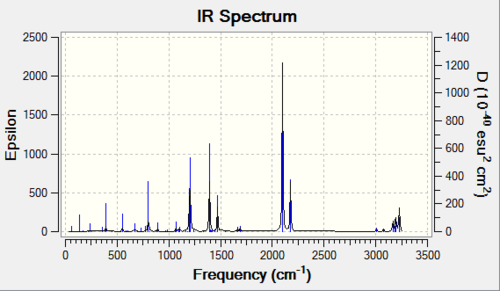
Imaginary frequency and Lowest frequency

IRC anaylsis
Job type: IRC, both directions, force constant once, calculate always, the number of points along the IRC= 50 Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
Gradient and energy change along the IRC claculation
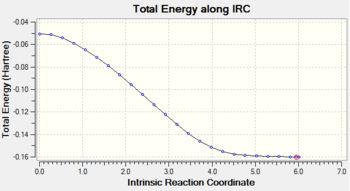
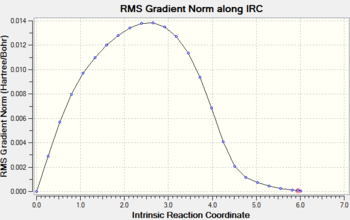
Endo transition state
Optimisation and frequncy anaylsis
Job type: Opt+Freq Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
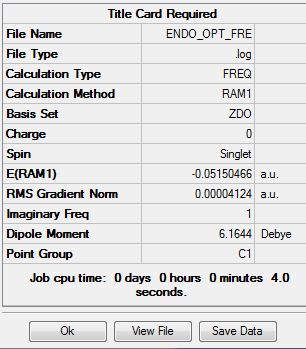
The output file confirm the calculation is converged.
Item Value Threshold Converged?
Maximum Force 0.000171 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.001704 0.001800 YES
RMS Displacement 0.000425 0.001200 YES
Predicted change in Energy=-1.429627D-07
Optimization completed.
-- Stationary point found.
The point group of this stucture after symmetrize is C1
HOMO and LUMO
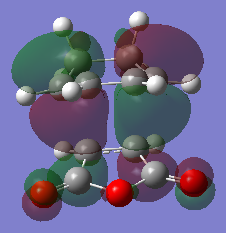
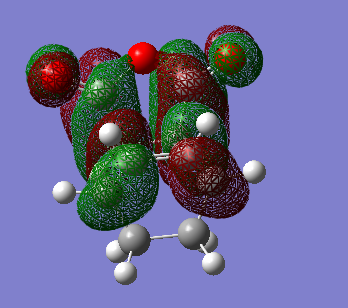
Predicted IR spectrum
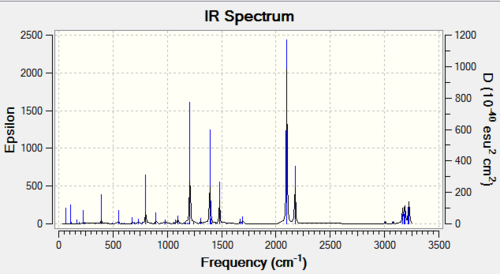
Imaginary frequency and Lowest frequency

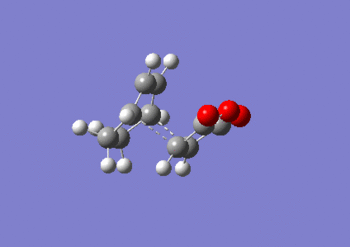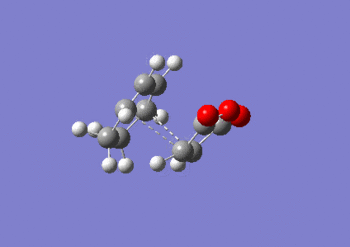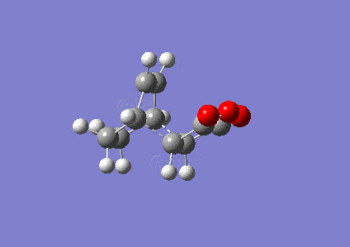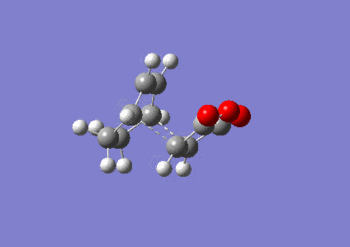
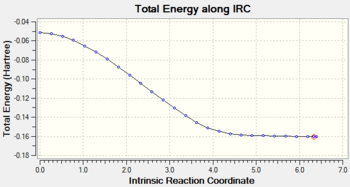
IRC anaylsis
Job type: IRC, both directions, force constant once, calculate always, the number of points along the IRC= 50 Method: semi-empirical Basis set: AM1
The log file of optimisation:click here
Gradient and energy change along the IRC claculation
Discussion
Compared to endo transition state, exo is higher in energy(exo -0.05041982 a.u. endo -0.05150466). The exo transition state is more strained due to the difference in the magnitude of the secondary interaction between carbon on the diene and the carbonyl carbon. For the molecular interaction, there are two fundamental interaction, primary, secondary. Primary interaction represents the molecular orbital interaction in which bond is forming or breaking, in other words active site of the reaction. Secondary interaction represents the molecular interaction between the atoms which are noe involved in the bond forming of breaking.In this case, compared to exo transition state, the carbon atoms on diene are closer to the carbonyl carbon in endo, which means a stronger secondary interaction and better orbital overlap . Therefore, the endo transition state is more satble.
For both transition states, there is only one imaginary vibration occurs and both vibration is perpendicular to the node planer. This vibration is related to the formation of the new carbon carbon sigma bond.
