Rep:Mod:Module1dc
Computational lab: Modelling using Molecular Mechanics
Introduction
Predictions of reactions are now possible using computational chemistry. Indeed, by using a molecular modelling, we are able to accurately model many aspects of organic structures and reactivity which appears to be extremely useful. Modelling can be made using Molecular Mechanics. This tool is particularly useful for studying specific properties of molecules such as strain, steric effects, stereoselectivity or conformation in an accurate manner. The main purpose of this module is to use Molecular Mechanics to predict and get a better understanding of the reactivity and the specificity of specific reactions. In fact, this method represents a considerable help in explaining the specific behaviour of molecules mainly by optimising the molecular geometry to a minimum and analyzing the bond strengh, Van Der Waals and dipole dipole interactions.
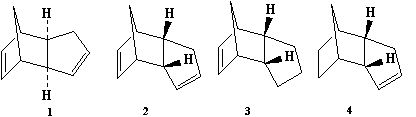
1. The Hydrogenation of Cyclopentadiene Dimer
Our first case of study will be the hydrogenation of the cyclopentadiene isomer. At room temperature, it often tends to dimerize to give the dicyclopentadiene compound. The dimerization occurs via a pericyclic type reaction, more specifically the Diels Alder reaction, giving rise to two dimers: endo (1) and exo (2) dicyclopentadiene. However, cyclopentadiene preferably dimerizes to give the endo dimer rather than the exo dimer. Additionally, considering the hydrogenation of the previous dimers, mainly due to steric hinderance reasons inducing further stability, we expect one major (4) and one minor (3) dihydro derivative products. By using results obtained from the molecular mechanics technique, we would be able to determine whether the dimerization of cyclopentadiene and its hydrogenation is thermodynamically or kinetically controlled.
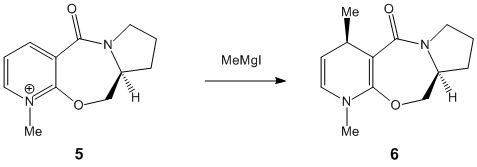
2. Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
This part of the module implies the investigation of two specific reactions. By minimising the models using the MM2 force field and analysing the data, we can effectively get a better understanding of the stereospecificity of the following two reactions. Additionally, the carbonyl group has an effect on the stereochemistry of the product. To explain it, we have to try several starting points for the optimization, in other words different geometry for the carbonyl group, and then compare the results.
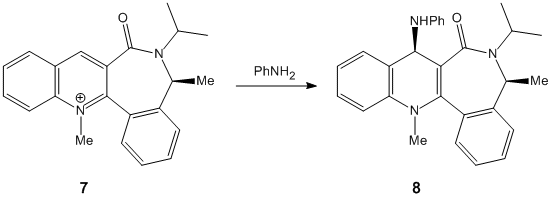
3. Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Concerning the Taxol compound, the geometry of the carbonyl group has an important effect on its stability. Indeed, in this part, by inducing slight modifications on the structure of each structure, we tried to obtain further minimization on the total energy of the molecule. The main modifications have been done on the structure of the six-membered ring but also on the specific geometry of the carbonyl group. Additionally, using the Molecular Mechanics methods, we will determine the most stable isomer and then rationalize why the alkene reacts slowly.

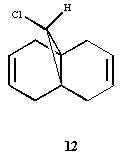
4. Regioselective Addition of Dichlorocarbene
Part 1 of this section is mainly designed to determine which of the two compound's alkene is the most nucleophilic. To do so, again, we need to optimise the compound's geometry and minimize the total energy of the molecule by using the Molecular Mechanics method in Chem3D. Also, we have to use the MOPAC/PM6 method to obtain a reasonnable approximate representation of the valence-electron molecular wavefunction, and more specifically of the HOMO (Highest Occupied Molecular Orbital).
Part 2 involves the calculation of the influence of the Cl-C bond on the vibrational frequencies of the studied molecule. We will then compare the actual compound and its hydrogenated derivative as well as recording the actual HOMO-1, the LUMO, LUMO+1 and LUMO+2 using the MOPAC/RM1 method. Finally, C=C stretches for the diene and C=C stretch for the monohydrogenated derivative have to be found and commented on their differences in values.
5. Structure based Mini project using DFT-based Molecular orbital methods
In this section, we had to choose a specific reaction from the litterature which produces two different isomers. By using different spectroscopic techniques, we would be able to compare the actual theorical values found using Molecular Mechanics with the experimental data. Spectroscopic techniques used are mainly 13C NMR and IR spectroscopy in order to differenciate the two different isomer products. Chem3D is mainly used to draw the two found isomers, the created files were submitted to the SCAN firstly for geometry optimization and then for NMR Chemical shift calculation. Additionally, we also predicted the IR spectrum of our compounds by calculating the vibrational normal modes of the molecules also via SCAN. For both data analysis, the output files from SCAN is opened in Gaussview, allowing us to predict the 13C NMR and the IR spetrum of the isomers.
Conclusion
Computational Chemistry is a useful tool for predicting reactions and the reactivity of specific species. Indeed, optimizing the geometry of the molecule to minimize its total energy and thus find the most favourable conformation for a reaction is effectively a exellent way of getting a better understanding on how and why reactions occur. However, computational methods has their limitations and errors. For instance, since it cannot reproducible exactly the same environment and conditions of reaction, the data will slightly deviate from experimental values found in labs.
