Rep:Mod:ECN0511MP
Name: Estella Chin Ning
CID: 00697238
Lewis Acids and Bases Mini Project
Introduction
This mini project aims to explore and analyse the conformers, vibrations and MOs of Al2Cl4Br2.
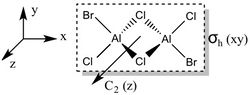
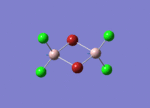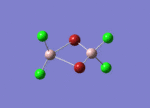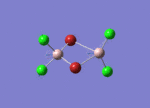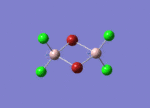
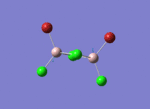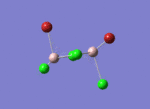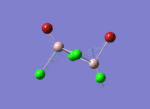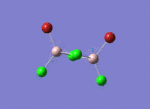
Firstly, the four possible Al2Cl4Br2 isomers that can be formed from AlCl2Br monomers were determined. They are:

Determining the Point Groups of each isomer
The point group symmetry of each isomer was then determined by identifying the symmetry elements present.
| Isomer 1 | Isomer 2 | Isomer 3 | Isomer 4 | |
|---|---|---|---|---|
| Structure | 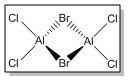
|
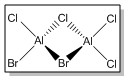
|
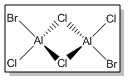
|
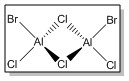
|
| Symmetry Elements | E, 3 х C2, 3 x σv, 3 x σh, 3 x S2, i
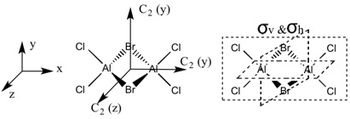 |
E | E, C2, σh, i | E, C2, 2 x σv |
| Point Group | D2h | C1 | C2h | C2v |
Optimisation
Optimisation of energies for each isomer was then determined by first using a 3-21G optimisation, followed by a 6-31G (d,p) optimisation, then a gen [full basis set 6-31G(d,p) on Al and Cl and a PP LANL2DZdp on Br] calculation to get the final optimised structure and energy. The results are presented below.
Isomer 1
3-21G Optimisation
The results of the Al2Cl4Br2 Isomer 1 3-21G optimisation are summarised in Table 2 below.
| Link to log file of 3-21G Optimised Al2Cl4Br2 Isomer 1 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Final energy | -7438.22199842 a.u. |
| RMS gradient norm | 0.00001806 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 18 minutes 14 seconds |
The expected point group symmetry of D2h is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000037 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.000987 0.001800 YES
RMS Displacement 0.000296 0.001200 YES
Predicted change in Energy=-2.344066D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
6-31G (d,p) Optimisation
The results of the Al2Cl4Br2 Isomer 1 6-31G (d,p) optimisation are summarised in Table 3 below.
| Link to log file of 6-31G (d,p) Optimised Al2Cl4Br2 Isomer 1 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G (d,p) |
| Final energy | -7469.54264936 a.u. |
| RMS gradient norm | 0.00003193 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 6 minutes 39.5 seconds |
The expected point group symmetry of D2h is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000117 0.000450 YES
RMS Force 0.000044 0.000300 YES
Maximum Displacement 0.001392 0.001800 YES
RMS Displacement 0.000557 0.001200 YES
Predicted change in Energy=-1.401266D-07
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Gen (6-31G (d,p) and LanL2DZ) Optimisation
The results of the Al2Cl4Br2 Isomer 1 Gen (6-31G (d,p) and LanL2DZ) optimisation are summarised in Table 4 below.
| Link to log file of Gen (6-31G (d,p) and LanL2DZ) Optimised Al2Cl4Br2 Isomer 1 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31G (d,p) and LanL2DZ) |
| Final energy | -2352.40630796 a.u. |
| RMS gradient norm | 0.00000777 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 3 minutes 58.2 seconds |
The expected point group symmetry of D2h is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000029 0.000450 YES
RMS Force 0.000011 0.000300 YES
Maximum Displacement 0.000682 0.001800 YES
RMS Displacement 0.000283 0.001200 YES
Predicted change in Energy=-1.422361D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Isomer 2
3-21G Optimisation
The results of the Al2Cl4Br2 Isomer 2 3-21G optimisation are summarised in Table 5 below.
| Link to log file of 3-21G Optimised Al2Cl4Br2 Isomer 2 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Final energy | -7438.22542072 a.u. |
| RMS gradient norm | 0.00001098 a.u. |
| Dipole moment | 1.66 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 16 minutes 12.3 seconds |
The expected point group symmetry of C1 is attained in the optimisation calculation above. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000016 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000799 0.001800 YES
RMS Displacement 0.000354 0.001200 YES
Predicted change in Energy=-7.627394D-09
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
6-31G (d,p) Optimisation
The results of the Al2Cl4Br2 Isomer 2 6-31G (d,p) optimisation are summarised in Table 6 below.
| Link to log file of 6-31G (d,p) Optimised Al2Cl4Br2 Isomer 2 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G (d,p) |
| Final energy | -7469.53985968 a.u. |
| RMS gradient norm | 0.00002265 a.u. |
| Dipole moment | 1.05 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 8 minutes 51.6 seconds |
The expected point group symmetry of C1 is attained in the optimisation calculation above. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000914 0.001800 YES
RMS Displacement 0.000320 0.001200 YES
Predicted change in Energy=-3.933296D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Gen (6-31G (d,p) and LanL2DZ) Optimisation
The results of the Al2Cl4Br2 Isomer 2 Gen (6-31G (d,p) and LanL2DZ) optimisation are summarised in Table 7 below.
| Link to log file of Gen (6-31G (d,p) and LanL2DZ) Optimised Al2Cl4Br2 Isomer 2 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31G (d,p) and LanL2DZ) |
| Final energy | -2352.41110039 a.u. |
| RMS gradient norm | 0.00005450 a.u. |
| Dipole moment | 0.13 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 3 minutes 33.6 seconds |
The expected point group symmetry of C1 is attained in the optimisation calculation above. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000085 0.000450 YES
RMS Force 0.000037 0.000300 YES
Maximum Displacement 0.001698 0.001800 YES
RMS Displacement 0.000689 0.001200 YES
Predicted change in Energy=-1.740389D-07
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Isomer 3
3-21G Optimisation
The results of the Al2Cl4Br2 Isomer 3 3-21G optimisation are summarised in Table 8 below.
| Link to log file of 3-21G Optimised Al2Cl4Br2 Isomer 3 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Final energy | -7438.22904525 a.u. |
| RMS gradient norm | 0.00002142 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 9 minutes 29.9 seconds |
The expected point group symmetry of C2h is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000046 0.000450 YES
RMS Force 0.000013 0.000300 YES
Maximum Displacement 0.001133 0.001800 YES
RMS Displacement 0.000378 0.001200 YES
Predicted change in Energy=-4.356919D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
6-31G (d,p) Optimisation
The results of the Al2Cl4Br2 Isomer 3 6-31G (d,p) optimisation are summarised in Table 9 below.
| Link to log file of 6-31G (d,p) Optimised Al2Cl4Br2 Isomer 3 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G (d,p) |
| Final energy | -7469.53758654 a.u. |
| RMS gradient norm | 0.00003242 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 8 minutes 43.2 seconds |
The expected point group symmetry of C2h is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000052 0.000450 YES
RMS Force 0.000024 0.000300 YES
Maximum Displacement 0.000617 0.001800 YES
RMS Displacement 0.000308 0.001200 YES
Predicted change in Energy=-7.219445D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Gen (6-31G (d,p) and LanL2DZ) Optimisation
The results of the Al2Cl4Br2 Isomer 3 Gen (6-31G (d,p) and LanL2DZ) optimisation are summarised in Table 10 below.
| Link to log file of Gen (6-31G (d,p) and LanL2DZ) Optimised Al2Cl4Br2 Isomer 3 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31G (d,p) and LanL2DZ) |
| Final energy | -2352.41629239 a.u. |
| RMS gradient norm | 0.00003186 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 3 minutes 37.6 seconds |
The expected point group symmetry of C2h is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000427 0.001800 YES
RMS Displacement 0.000169 0.001200 YES
Predicted change in Energy=-1.597545D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Isomer 4
3-21G Optimisation
The results of the Al2Cl4Br2 Isomer 4 3-21G optimisation are summarised in Table 11 below.
| Link to log file of 3-21G Optimised Al2Cl4Br2 Isomer 4 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Final energy | -7438.22885199 a.u. |
| RMS gradient norm | 0.00003640 a.u. |
| Dipole moment | 2.1693 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 20 minutes 43.6 seconds |
The expected point group symmetry of C2v is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000079 0.000450 YES
RMS Force 0.000026 0.000300 YES
Maximum Displacement 0.001760 0.001800 YES
RMS Displacement 0.000594 0.001200 YES
Predicted change in Energy=-5.836772D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
6-31G (d,p) Optimisation
The results of the Al2Cl4Br2 Isomer 4 6-31G (d,p) optimisation are summarised in Table 12 below.
| Link to log file of 6-31G (d,p) Optimised Al2Cl4Br2 Isomer 4 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G (d,p) |
| Final energy | -7469.53765574 a.u. |
| RMS gradient norm | 0.00003383 a.u. |
| Dipole moment | 1.1525 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 7 minutes 36.0 seconds |
The expected point group symmetry of C2v is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000053 0.000450 YES
RMS Force 0.000025 0.000300 YES
Maximum Displacement 0.001133 0.001800 YES
RMS Displacement 0.000329 0.001200 YES
Predicted change in Energy=-8.040274D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Gen (6-31G (d,p) and LanL2DZ) Optimisation
The results of the Al2Cl4Br2 Isomer 4 Gen (6-31G (d,p) and LanL2DZ) optimisation are summarised in Table 13 below.
| Link to log file of Gen (6-31G (d,p) and LanL2DZ) Optimised Al2Cl4Br2 Isomer 4 | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31G (d,p) and LanL2DZ) |
| Final energy | -2352.41628070 a.u. |
| RMS gradient norm | 0.00001475 a.u. |
| Dipole moment | 0.15 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 5 minutes 4.0 seconds |
The expected point group symmetry of C2v is not attained in the optimisation calculation above. This is because higher accuracy calculations are required to attain the correct point group symmetry of the Al2Cl4Br2 molecule. In addition, the gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000040 0.000450 YES
RMS Force 0.000016 0.000300 YES
Maximum Displacement 0.001320 0.001800 YES
RMS Displacement 0.000411 0.001200 YES
Predicted change in Energy=-2.520994D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Optimisation Summary
The comparison of the results from the final optimisation step (Gen: 6-31G (d,p) and LanL2DZ) between the 4 Al2Cl4Br2 isomers are summarised in Table 14 below.
Discussion: Position of Br atoms w.r.t stability of different conformers
Comparing the final energies of the conformers, the stability of the 4 different isomers can be ranked in the order (from least stable to most stable):
The difference in stabilities can be rationalised by the position of the Br atoms in the conformers:
1. Whether the Br atoms are bridging or terminal atoms
2. If the 2 Br atoms are terminal atoms (Isomers 3 and 4), whether the Br atoms are cis or trans to each other
1. Bridging or terminal Br atoms
When a Br atom is in a bridging position, it forms 2 Al - Br bonds as compared to 1 Al - Br bond when it is in a terminal position. Since a Br atom has a relatively larger size as compared to a Cl atom, it has more diffuse atomic orbitals, and hence form a less effective overlap with the atomic orbitals of the Al atom, resulting in the formation of a weaker Al - Br bond as compared to a Al - Cl bond. This means that a bridging Br atom (2 x weak Al - Br bonds) will result in a higher energy, less stable conformer as compared to a bridging Cl atom (2 x strong Al - Cl bonds). This effect is additive; the more the number of bridging Br atoms, the more the number of Al - Br bonds, the less stable the conformer. Comparing the different isomers, isomer 1 has 2 bridging Br atoms and 4 x Al - Br bonds, isomer 2 has 1 bridging Br atom, 1 terminal Br atom and 3 x Al - Br bonds, while both isomers 3 and 4 have 0 bridging Br atoms, 2 terminal Br atoms and 2 Al - Br bonds. This results in the observed trend of relative stabilities Isomer 1 < Isomer 2 < Isomers 3 & 4.
2. Cis or trans Br atoms
Although both isomers 3 and 4 do not contain bridging Br atoms, isomer 3 is slightly more stable than isomer 4, which can be explained by the arrangement of the terminal Br atoms relative to each other. Isomer 3 contains the Br atoms in a trans arrangement (opposite sides), while isomer 4 contains the Br atoms arranged in a cis arrangement (same side). Consequently, isomer 3 has no net dipole moment as the dipole moments cancel each other as compared to isomer 4 which has a net dipole moment of 0.15 D (c.f. Table 13). Non-polar molecules are relatively more stable than their polar isomers as they are less reactive. Hence, this gives us the observed trend of relative stabilities Isomer 1 < Isomer 2 < Isomers 4 < Isomer 3.
Dissociation Energy for Isomer 3
The dissociation energy for the lowest energy conformer of Al2Cl4Br2 (Isomer 3) into 2 AlCl2Br monomers were then computed. This was done by first optimising the energies of a AlCl2Br molecule in the same way as before (3-21G, 6-31G (d,p), gen (6-31 (d,p) and LanL2DZ)).
Optimisation of AlCl2Br Monomers
3-21G Optimisation
The results of the AlCl2Br 3-21G optimisation are summarised in Table 15 below.
| Link to log file of 3-21G Optimised AlCl2Br | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 3-21G |
| Final energy | -3719.09049394 a.u. |
| RMS gradient norm | 0.00009870 a.u. |
| Dipole moment | 1.13 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 3 minutes 37.1 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000148 0.000450 YES
RMS Force 0.000082 0.000300 YES
Maximum Displacement 0.001216 0.001800 YES
RMS Displacement 0.000823 0.001200 YES
Predicted change in Energy=-1.795635D-07
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
6-31G (d,p) Optimisation
The results of the AlCl2Br 6-31G (d,p) optimisation are summarised in Table 16 below.
| Link to log file of 6-31G (d,p) Optimised AlCl2Br | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | 6-31G (d,p) |
| Final energy | -33.74850546 a.u. |
| RMS gradient norm | 0.00003286 a.u. |
| Dipole moment | 0.69 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 2 minutes 4.5 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000121 0.000450 YES
RMS Force 0.000065 0.000300 YES
Maximum Displacement 0.001490 0.001800 YES
RMS Displacement 0.000940 0.001200 YES
Predicted change in Energy=-1.514838D-07
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Gen (6-31G (d,p) and LanL2DZ) Optimisation
The results of the AlCl2Br Gen (6-31G (d,p) and LanL2DZ) optimisation are summarised in Table 17 below.
| Link to log file of Gen (6-31G (d,p) and LanL2DZ) Optimised AlCl2Br | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FOPT |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31G (d,p) and LanL2DZ) |
| Final energy | -1176.19013709 a.u. |
| RMS gradient norm | 0.00001883 a.u. |
| Dipole moment | 0.11 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 1 minutes 22.0 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Item Value Threshold Converged?
Maximum Force 0.000041 0.000450 YES
RMS Force 0.000025 0.000300 YES
Maximum Displacement 0.000401 0.001800 YES
RMS Displacement 0.000244 0.001200 YES
Predicted change in Energy=-1.323149D-08
Optimization completed.
-- Stationary point found.
The table above shows that the forces have converged, meaning that for a small displacement, the energy does not change. This further confirms the successful optimisation of the molecule.
Calculation of Dissociation Energy
The comparison table of reaction energies between AlCl2Br and Al2Cl4Br2 can be found in Table 18 below.
| E (AlCl2Br) / a.u. | - 1176.19013709 |
|---|---|
| E (Al2Cl4Br2, Isomer 3) / a.u. | - 2352.41629239 |
| Energy Difference/ a.u. | - 2352.41629239 - ( - 1176.19013709 - 1176.19013709 ) = - 0.036018 |
| Energy Difference = Association Energy / kJmol-1 | - 0.036018 x 2625.50 = - 94.565259 = - 90 (Accuracy up to 10kJ/mol) |
| Dissociation Energy / kJmol-1 | 94.565259 = - 90 (Accuracy up to 10kJ/mol) |
Discussion: Comparison of Reactant and Product Stabilities
The product is more stable than the reactant. This can be inferred from the negative association energy, meaning that the combination of 2 AlCl2Br molecules to form isomer 3 of Al2Cl4Br2 is an exothermic process involving the release of energy. In the same way, since the dissociation energy is positive, this suggests that the breaking up of the Al2Cl4Br2 dimer molecule into 2 AlCl2Br monomer molecules is an endothermic process, i.e., energy is required to convert the more stable Al2Cl4Br2 dimer molecule into 2 less stable AlCl2Br monomer molecules.
Frequency Analysis
The frequency analysis for each conformer was then calculated and the results presented below.
Isomer 1
The results of the frequency calculations are summarised in Table 19 below.
| Link to log file of Isomer 1 Frequency analysis | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FREQ |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31 (d,p) and LanL2DZ) |
| Final energy | -2352.40630796 a.u. (Same (up to last 2 d.p.) as that recorded in optimisation step) |
| RMS gradient norm | 0.00000777 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 4 minutes 16.1 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Low frequencies --- -5.1406 -5.0830 -3.1891 -0.0024 -0.0014 0.0008 Low frequencies --- 14.8596 63.2584 86.0498
The low frequencies are within the range of ± 15 cm-1 and close to 0, indicating that the method employed in the frequency calculations was sufficiently accurate.
Isomer 2
The results of the frequency calculations are summarised in Table 20 below.
| Link to log file of Isomer 2 Frequency analysis | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FREQ |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31 (d,p) and LanL2DZ) |
| Final energy | -2352.41110039 a.u. (Same (up to last 2 d.p.) as that recorded in optimisation step) |
| RMS gradient norm | 0.00005448 a.u. |
| Dipole moment | 0.13 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 4 minutes 4.3 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Low frequencies --- -3.6216 -2.0558 -0.0006 0.0007 0.0029 3.2124 Low frequencies --- 17.3982 55.7388 80.0202
The low frequencies are within the range of ± 15 cm-1 and close to 0, indicating that the method employed in the frequency calculations was sufficiently accurate.
Isomer 3
The results of the frequency calculations are summarised in Table 21 below.
| Link to log file of Isomer 3 Frequency analysis | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FREQ |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31 (d,p) and LanL2DZ) |
| Final energy | -2352.41629239 a.u. (Same (up to last 2 d.p.) as that recorded in optimisation step) |
| RMS gradient norm | 0.00003185 a.u. |
| Dipole moment | 0.00 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 4 minutes 25.2 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Low frequencies --- -3.1697 -2.0334 0.0017 0.0024 0.0027 1.4953 Low frequencies --- 17.8868 49.0567 72.9517
The low frequencies are within the range of ± 15 cm-1 and close to 0, indicating that the method employed in the frequency calculations was sufficiently accurate.
Isomer 4
The results of the frequency calculations are summarised in Table 22 below.
| Link to log file of Isomer 4 Frequency analysis | |
|---|---|
| Link to D-Space | |
| File Type | .log |
| Calculation type | FREQ |
| Calculation method | RB3LYP |
| Basis set | Gen (6-31 (d,p) and LanL2DZ) |
| Final energy | -2352.41628070 a.u. (Same (up to last 2 d.p.) as that recorded in optimisation step) |
| RMS gradient norm | 0.00001475 a.u. |
| Dipole moment | 0.17 D |
| Point group | C1 |
| Time taken for calculation | 0 days 0 hours 4 minutes 26.4 seconds |
The gradient is less than 0.0001 (expected gradient = 0 for a stationary point), indicating that the molecule has indeed been optimised.
Low frequencies --- -2.2625 -1.6206 -0.0032 -0.0010 0.0012 3.6729 Low frequencies --- 17.0797 51.2950 78.5578
The low frequencies are within the range of ± 15 cm-1 and close to 0, indicating that the method employed in the frequency calculations was sufficiently accurate.
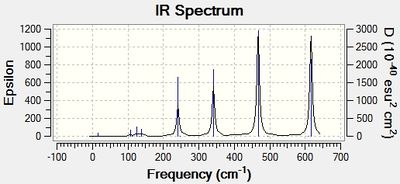
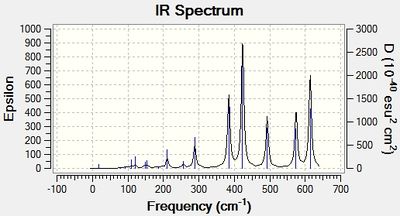
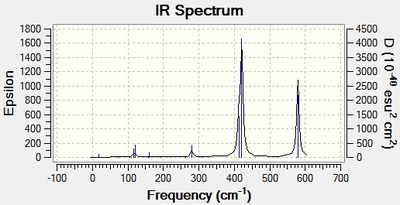
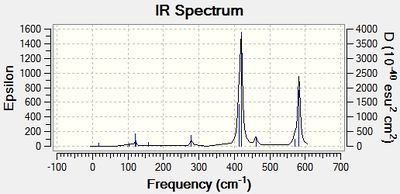
Infrared (IR) Spectra
Discussion: Conformer symmetry & No. of IR peaks
The number of bands that are visible in a IR spectrum is directly related to the number of vibrations which give rise to a change in the net dipole moment of the molecule. That is, a vibration is only IR active when it is associated with a change in the net dipole moment of the molecule. When 2 or more different vibrations are associated with the same energy, they give rise to degenerate peaks and hence only 1 peak shows up in the IR spectrum. However, for the 4 different isomers of Al2Cl4Br2, no degenerate peaks were detected in the calculations, hence the difference in the number of IR peaks in the 4 spectra can be attributed to the difference in the number of vibrations which cause a change in the net dipole moment of each conformer.
The higher the symmetry of the isomer, the more the number of symmetrical stretching and bending vibrational modes. These vibrations do not change the net dipole moment of the molecule, giving rise to IR peaks with zero intensity that do not show up in the IR spectrum. Thus, more symmetrical molecules have fewer bands in their IR spectrum. This is corroborated by the difference in the number of IR peaks observed in the table above; the most symmetrical isomer (isomer 1) has the fewest number of IR peaks (most peaks with zero intensity), while the least symmetrical isomer (isomer 2) has the most number of IR peaks (no peaks with zero intensity).
Comparing Vibrational Frequencies
Isomer 1
Isomer 2
- The nature of the Al - Br stretching cannot be classified into symmetrical and asymmetrical stretching as the molecule is not a symmetrical one.
Isomer 3
Isomer 4
Comparison of Al-Br Vibrational Frequencies
Discussion: Comparison of Key Vibrational Frequencies
As can be inferred from Table 28, Isomer 1, whose 4 key vibrational modes all entails the stretching of the Al-bridging Br bonds, have a lower Al-Br stretch frequency position (11-14) and corresponding frequency range (197-341 cm-1) as compared to Isomers 3 and 4, whose 4 key vibrational modes all entails the stretching of the Al-terminal Br bonds (Al-Br stretch frequency position: 15-18; Corresponding frequency range: 421-582 (Isomer 3) and 420-582 (Isomer 4)). This can be rationalised by the fact that the bridging Al-Br bonds are weaker than the terminal Al-Br bonds. The reason for this phenomenon is that the terminal Al-Br bond consists of a normal 2C-2e covalent bond, whereby the terminal Br atom only has to share 1 electron, while the bridging Al-Br bonds consist of 1 normal Al-Br covalent bond and 1 dative Al-Br bond. Since the electronegative Br atom now has to share 3 of its electrons over 3 atoms (Al-Br-Al fragment), this results in weaker bridging Al-Br bonds relative to terminal Al-Br bonds. A weaker Al-Br bond results in a lower Al-Br bond strength and corresponding lower force constant (k), which is directly proportional to the frequency of the vibration. Hence, bond stretching involving terminal Br atoms will have higher frequencies as compared to those involving bridging Br atoms. As such, a lower vibration frequency range can be expected for isomer 1 as compared to isomers 3 and 4, which corroborates with the frequency calculations.
Isomer 2, which contains 2 vibrational modes from the Al-terminal Br bond stretching and 2 vibrational modes from the Al-bridging Br bond stretching, has Al-Br stretch frequency positions that falls within the range of both the Al-bridging Br stretching and Al-terminal Br stretching (11,14 (bridging range) 15,17 (terminal range)). This trend is also observed in its corresponding frequency range; the frequencies 211 cm-1 and 385 cm-1 fall within the bridging range, while the frequencies 423 cm-1 and 574 cm-1 fall within the terminal range. In conclusion, these findings show that the computational calculations provide frequency results that correspond with what we would expect theoretically.
Molecular Orbitals of Al2Cl4Br2 (Isomer 3)
A MO Calculation of the lowest energy conformer of Al2Cl4Br2 (Isomer 3) was then carried out, and the relevant links to the log file and the d-space can be found below.
Link to log file
Link to D-Space
The occupied, non-core MO structures of Al2Cl4Br2 (Isomer 3) are depicted in Figure 2.
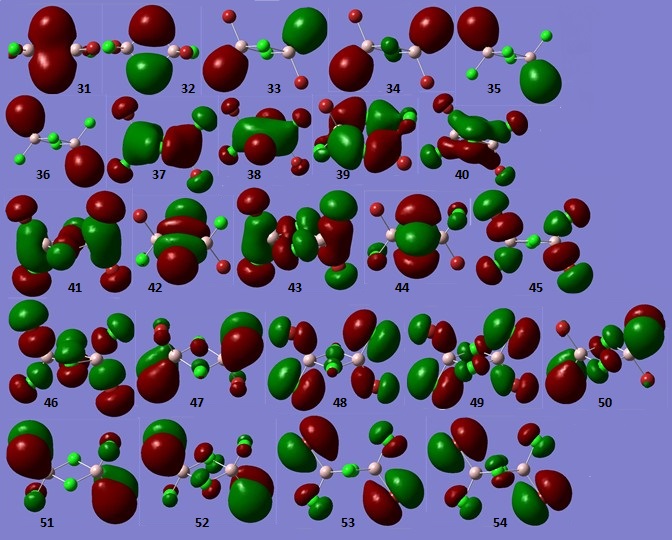
5 MOs ranging from highly bonding to highly antibonding were then chosen, and the interactions occuring in those MOs were analysed and described in Table 29.