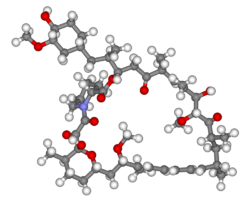
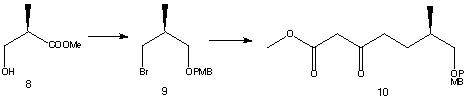Rapamycin
|
Cyclopentasiloxane |
Rapamycin, also known as Sirolimus, is a peptide that was isolated in 1975 from the bacteria strain Streptomyces hygroscopicus found in a soil sample on Easter Island. It is a macrolide, hence accounting for the "-mycin" in its name.
Rapamycin has been discovered to behave interestingly, possessing a novel mechanism of immunosuppression. Its mode of action differs largely from the other immunosupressants available, bearing great promise for its potential uses and advantages over other treatments.It is currently used as a new immunosuppressant drug, adminstered to precent rejection during organ transplants, particularly kidney transplants. It received approval from the FDA in September 1999, and has since been marketed as an immunosuppresant under the tradename 'Rapamune' by Wyeth-Ayerest.
The general shortage of organs available for transplants spells greater need for adrug that boosts the chance of organ survival. Conventional treatments used, such as cyclosporin and FK506, are effective in ensuring the short-term survival of the transplant, but fail in ensuring the organ is accepted by the body in the long run. Rapamycin is hence very important in the treatment of organ transplant patients as it appears to have a different mechanism of action to cyclosporin and FK506, as discussed earlier. In addition,it results in fewer side effects than the standard anti-rejection treatments due to its novel mode of action. Finally, the cytotoxic properties of Rapamycin could also make it effective in the treatment of cancer as Rapamycin is antiproliferative in nature, and there is ongoing research in this field of medicine.
| Properties of Rapamycin | |
|---|---|
[[Image: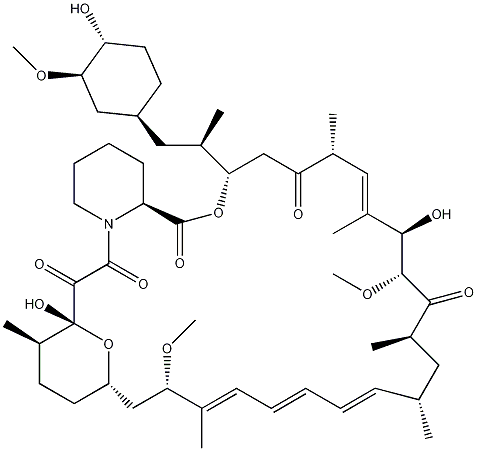 |250px|Properties of Rapamycin]] |250px|Properties of Rapamycin]]
| |
| IUPAC Systematic name | |
| (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,
26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26, 27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3- [(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]- 1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26- hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4]- oxaazacyclohentriacontine-1,5,11,28,29 (4H,6H,31H)-pentone | |
| Other name | |
| Sirolimus&btnG=Google+Search&meta= Rapamune, Sirolimus | |
| Indentifiers | |
| ATC Code | L04AA10 |
| CAS number | {{{CASNo}}} |
| PubChem (CID) | 6436030 |
| SMILES | [C@@H1CC[C@@H](C[C@@H](C)[C@H](CC([C@H](C)/C=C([C@H]
([C@H](C([C@@H]C[C@H](C)/C=C/C=C/C=C(C)/[C@@H](OC)C[C@@H]2CC[C@@H] (C)[C@@](C(C(N4[C@H]3CCCC4)=O)=O)(O)O2)C)=O)OC)O)\C)=O)O[C@@]3=O) C[C@H]1OC O[C@@H]1CC[C@@H](C[C@@H](C)[C@H](CC([C@H](C)/C=C([C@H] ([C@H](C([C@@H]C[C@H](C)/C=C/C=C/C=C(C)/[C@@H](OC)C[C@@H]2CC[C@@H] (C)[C@@](C(C(N4[C@H]3CCCC4)=O)=O)(O)O2)C)=O)OC)O)\C)=O)O[C@@]3=O) C[C@H]1OC] |
| Chemical Data | |
| Molecular formula | C54H79NO13 |
| Molar mass | 914.172 g/mol g/mol |
| Pharmacokinetic Data | |
| Bioavailability | 20%, decreases after consumption of food rich in fat |
| Protein Binding | 92% |
| Metabolism | Hepatic |
| Half life | 57 - 63 hours |
| Excretion | Mostly faecal |
| Therapeutic considerations | |
| Pregnancy cat. | C(AU) C(US) |
| Legal status | Rr only (US) |
| Routes | Oral |
Optical Rotary Power
Type: Alpha
Optical Rotary Power: -58.2 degrees
Wavelength: 589 nm
Temperature: 298.15 K
Appearance
Off-white to slight yellow powder
Purity
At least 97% by TLC, HPLC
Melting point
178°C-182°C
Solubility
Rapamycin (Sirolimus) gives clear colorless solution at 50mg/ml DMSO
Storage
-20°C. Protect from light and moisture. Hygroscopic
Numerous total synthese of Rapamycin have been reported, on top of many part- and fragments- syntheses.
Rapamycin is a complex molecule, containing a 31-membered ring which includes a pipecolinyl group and pyranose ring, a conjugated triene system and a tri-carbonyl region. It also has 15 chiral centres, suggesting that the number of possible stereoisomers is enormous. The synthesis of Rapamycin hence poses a great challenge to synthetic chemists.
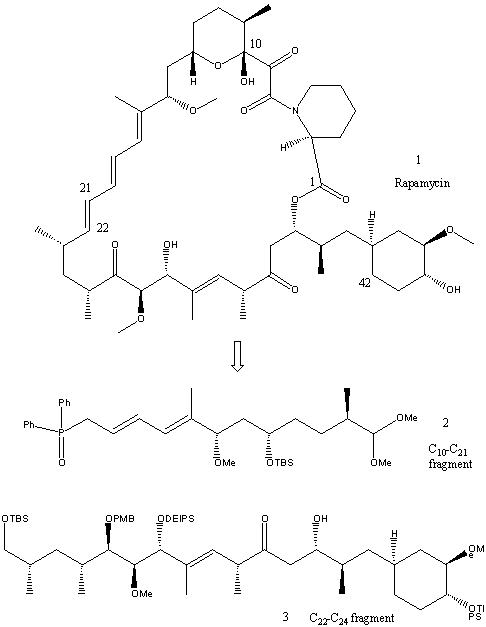
In the following synthesis, published in three separate papers, two fragments of C10-C21 and C22-C42 are formed separately, before being combined to give the total synthesis of rapamycin. Only the main outline of the synthesis will be illustrated on this page as it is too long and complex to show in great detail.
Retro-synthesis
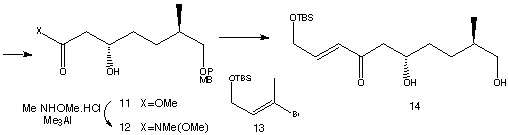
In the retro-synthesis shown below, the molecule is disconnected at the ester group next to Carbon 1and the C21 - C22 double bond of the triene, producing the synthetic precursors 2 and 3. Further disconnections of 3 will be shown later. The C10-C21 fragment is first synthesised.
1. Synthesis of C10-C21 fragment
The starting material of synthesis is (R)-methyl 3-hydroxy-2-methylpropionate (8).
The starting material (8) is then converted to an alcohol in a four-step process.
1. Protection of the alcohol as aTHP ether, followed by
2. Reduction,
3. Ether formation and finally,
4. Deprotection steps.
Bromide (9) is formed with the substitution of the hydroxyl group in the product with a bromine. Subsequent reaction of (9) with methyl acetoacetate produced an ester, (10) .
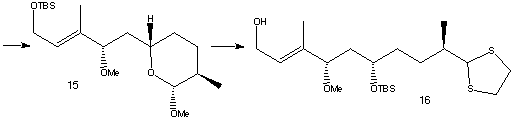
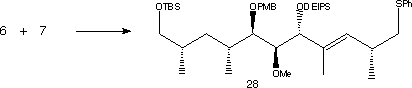
Catalytic reduction of (10) under Noyori conditions yielded ester (11), which is later converted to its Weinreb amide (12). Overall, percentage yield of compound (12) is 54%, from a relatively cheap starting material. Vinyl bromide, (13) was then metallated with t-BuLi and the resulting vinyllithium was then combined with (12) and the PMB-protecting group removed to produce (14). The remaining carbonyl group in (14) was selectively reduced to a hydroxyl group. In order to differentiate the 1,3-diol, a lactol was formed, where one hydroxyl group ended up in the ring. An oxidation was performed using RuCl2(PPh3)3 to form a lactol. The two remaining alcohol groups can then be methylated using MeI to give (15).
The lactol ring opening was achieved using TiCl4 and thiol HS(CH2)2SH to form a dithiolane. The freed alcohol was then protected as its TBS ether and the same protecting group selectively removed from the primary alcohol to form (16). To avoid removing the dithiolane group at a later stage in the synthesis, the thio-acetal was converted to the dimethyl acetal (17) using PhI(OCOCF3)2 and methanol.
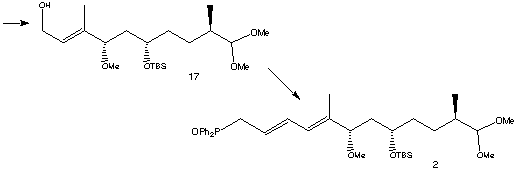
The next stage in the synthesis was to extend (17) for the building of the triene region. The terminal alcohol was oxidised to its aldehyde using BaMnO4 , then a Wittig reaction was carried out using Ph3P=CHCO2Et and CH2Cl2 to form the second double bond. Reduction of the ester group to an alcohol was carried out using DIBAL-H, then treatment with PPh3. Susequent exposure to the air gave Rapamycin fragment 2.
2. Synthesis of C22-C42 fragment
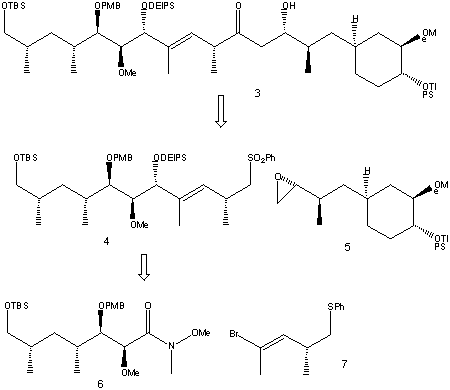
Retrosynthesis of (3)gives the three synthetic precursors 5, 6 and 7. It was thought (4) could be obtained by alkylative coupling of a vinyllithium species generated from (7) to the Weinreb amide (6). The nucleophilic opening of epoxide (5) by the lithiated sulfone from phenyl sulfone (4) would then give the desired fragment.
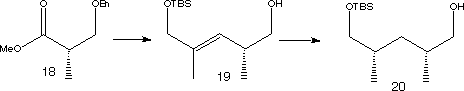
The ester (18) was used as a starting material to make fragment (6).
A Wittig reaction, followed by reduction and protection steps, give (19). This was hydrogenated using a rhodium catalyst to give syn-dimethyl product (20). The minor anti diastereomer was successfully separated off. (20) was oxidised, before undergoing an aldol condensation to give adduct (21).
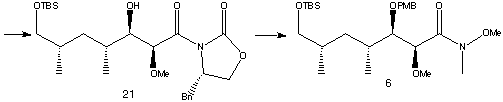
Transamination of (21)and protection of the alcohol with PMB produced amide (6) text, corresponding to the C22-C28 segment of Rapamycin.
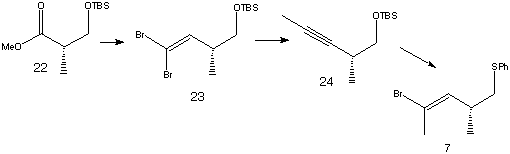
The vinyl bromide (7) was prepared using ester (22) as a starting material.
Reduction of (22), followed by dibromoolefination, led to product (23). Acetylene (24) was prepared using n-BuLi, THF and MeI, then sulfenylation with Ph2S2 and bromination gave fragment (7).
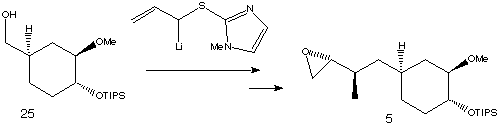
Iodination and alkylation of starting material (25) with the lithiated allylic sulfide shown followed by a number of further steps, resulted in its conversion to fragment (5).
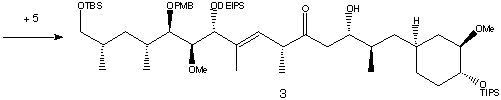
Fragments (7) was first converted to its vinyllithium using t-BuLi, then combined with (6) to form an enone of 78% yield. Stereoselective reduction of the carbonyl group using Zn(BH4)2 gave an alcohol which was protected with DEIPS giving (28). The phenyl sulfide was oxidised to a sulfone using m-CPBA in excess pyridine.
Lithiation and addition of the epoxide (50 resulted in the hydroxy sulfone in a 4:1 ratio of two diastereomers, which were separated by HPLC. Metalation using n-BuLi followed by oxidation formed the total C22-C42 fragment.
3. Total Synthesis of Rapamycin using combination of C10-C21 and C22-C42 fragments
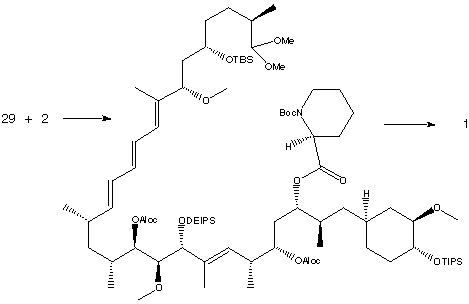
Fragment (3) (C22-C42) was treated with (S)-Boc-pipecolinal, followed by a Swern oxidation, yielded the aldehyde (29).
Condensation with the lithium salt of phosphine oxide 2 (C10-C21) produced the triene as shown below.
The triene was hydrolysed with pyridinium p-toluenesulfonic acid and an aldol reaction was performed. Treatment with triethylsilyl triflate produced an amino acid, which was subjected to Mukaiyama macrocyclization conditions to form the 31-membered ring.
Finally, deprotection steps were performed to give synthetic Rapamyin (1). The identity of this Rapamycin sample is confirmed by comparison of physical properties, 1H-NMR, 13C-NMR, IR and UV spectral data.
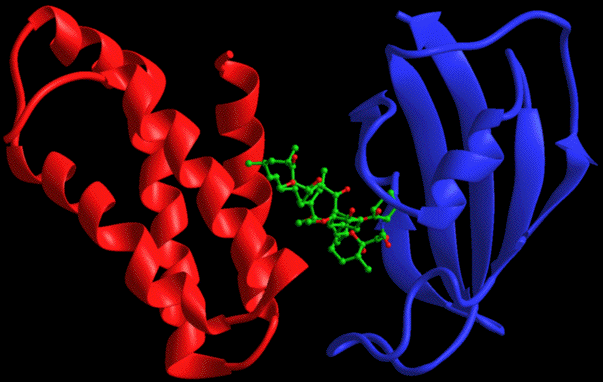
Rapamycin is believed to block the immune response by causing programmed cell death, otherwise known as apoptosis, in T cells. Rapamycin penetrates the cell membrane of T-cells and binds to an intracellular receptor called FKBP (FK506 Binding Protein). This complex then binds to FRAP (FKBP Rapamycin Associated Protein), a regulator of the G1 phase of the cell cycle.
The diagram below illustrates the complex binding, where FKBP-12 is represented by the blue protein and FRAP the red protein, with Rapamycin between them.
This Rapamycin complex inhibits the T cell response to IL-2, the substance which triggers T cells already activated by the TCR to progress through G1 of the cell cycle. Rapamycin hence stops the cell at the G1-S transition. As such, the proliferation of T-cells is stopped and apoptosis is induced instead.
Activation of T cells produce a small population of regulatory T cells, which possess the abilibty to control the other T cells that cause rejection. The apoptic death of the many rejection-causing T cells enables the regulatory T cells to override the rejection process. Rapamycin blocks the proliferation of activated T cells though it does not block apoptosis.Therefore, by inducing apoptosis in rejection-causing T cells, Rapamycin can reduce the tendency to reject the transplant, yet allowing the body to develop a tolerance for it.
Cell division is controlled by cyclin dependent kinases, cyclins and p53, a protein which blocks the cell cycle if the DNA is damaged, leading to apoptosis. Usually, cancer is caused by a p53 mutation, where abnormal cells are prevented from dying by apoptosis. Instead they will continue to divide uncontrollably, reproducing and magnifying the error. Rapamycin could cease the division of cancer cells in a similar fashion in which it stops cell division in T-cells, and also cause apoptosis. It could hence be adminstered in the treatment of cancer.
Studies have revealed that Rapamycin is capable of inhibiting growth and induce cell death by apoptosis in B lymphoma cells.