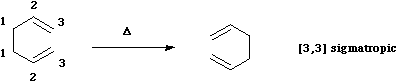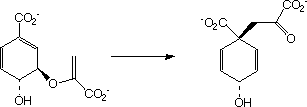Organic:pericyclic:examples
Appearance
Examples Of Electrocyclic Reactions.
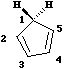
- Opening of the cyclopropyl cation involves only one electron arrow, and hence the number of cyclically conjugated electrons is 2 (4n+2, n=0). Under thermal conditions, this will go via a Huckel topology involving suprafacial components., which has the effect of rotating both methyl groups outwards in opposite directions (disrotation).
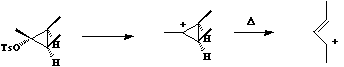
- Both the reactions below involve two electron arrows and hence are 4n systems (n=1).
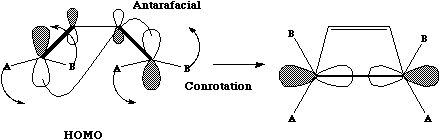
 Thermally, they will proceed via a Mobius topology involving one antarafacial component. Both methyl groups will rotate in the same direction (conrotation) leaving one endocyclic and one exocyclic. This can also be (optionally) illustrated via the Frontier orbital approach.
Thermally, they will proceed via a Mobius topology involving one antarafacial component. Both methyl groups will rotate in the same direction (conrotation) leaving one endocyclic and one exocyclic. This can also be (optionally) illustrated via the Frontier orbital approach.
In order to form a new sigma bond, the HOMO of the butadiene fragment must rotate in the sense shown, one node coming from above the plane of the molecule, the other from below [#map (ie antarafacial).] - Under photochemical conditions, a 4n reaction is predicted to proceed via a Huckel topology with suprafacial bond formation, as for example
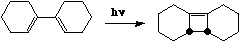 The sense of this can again be seen from the frontier orbital (normally the LUMO but in the excited state occupied by a single electron, and hence effectively the HOMO).
The sense of this can again be seen from the frontier orbital (normally the LUMO but in the excited state occupied by a single electron, and hence effectively the HOMO).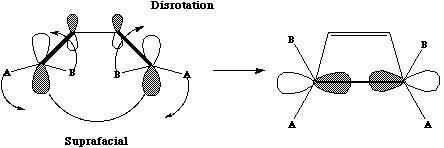
- Where heteroatoms are involved, the lone pair counts as two electrons. Thus the ring opening of aziridine is a 4 electron process, hence 4n proceeding via Mobius topology with both methyl groups rotating in the same direction (conrotation).
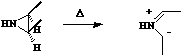
- One important aspect when counting electrons is to include only the cyclically conjugated system. In the example below, for one resonance isomer of the [16] annulene shown, the arrow pushing gives rise to two independently cyclic six electron (4n+2) reactions, and the electrons in the central double bonds are NOT counted in the process, since they are apparently uninvolved in the reaction;
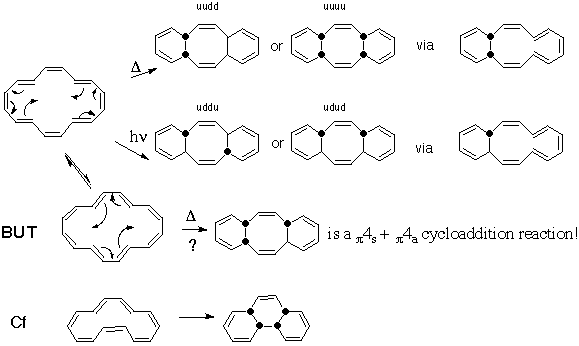 This reaction is however full of subtle complexities. The original report of the thermal reaction implied the product was the one marked as uudd. In fact, another isomer, uuuu is also allowed by the selection rules, and molecular modelling suggests this isomer is in fact significantly more stable (by about 12 kJ/mol). Similarly, the photochemical reaction, which follows a Mobius transition state, can give rise to both uddu and udud. The latter is again predicted the more stable by molecular modelling by about 12 kj/mol. Because two (independent) pericyclic processes are involved, this implies in each case the intermediacy of molecules in which only one C-C bond has been formed. TWO pericyclic transition states are thus involved in this reaction. Molecular modelling implies the [#map first bond formation has a much higher energy then the subsequent second one] (by about 78 kJ/mol). Click on the diagram above to see models generated using molecular modelling. If instead, the [16]annulene is considered as a bond valence isomer (Note, this is NOT the same as a resonance isomer), then it adopts an entirely different shape from the other isomer. In fact, the first isomer has a plane of symmetry and the second isomer an exis of symmetry, but also a higher energy. With this new shape an alternative way of forming the two bonds can be drawn, which involves pushing one set of four arrows, and thus constitutes a single pericyclic 12 electron (4n) process formally equivalent to a cycloaddition reaction, but with the selection rules predicting a quite different stereochemical outcome! Here, the predictions of theory can be tested by observing the stereochemistry of the final product. [Very recently, another, lower energy, [#map isomer] with an axis of symmetry has been located]. A brain teaser is how to consider the analogous reaction of the [14]annulene, where both the two separate electrocyclic reactions AND the concurrent cycloaddition reaction actually now predict the same stereochemical outcome!
This reaction is however full of subtle complexities. The original report of the thermal reaction implied the product was the one marked as uudd. In fact, another isomer, uuuu is also allowed by the selection rules, and molecular modelling suggests this isomer is in fact significantly more stable (by about 12 kJ/mol). Similarly, the photochemical reaction, which follows a Mobius transition state, can give rise to both uddu and udud. The latter is again predicted the more stable by molecular modelling by about 12 kj/mol. Because two (independent) pericyclic processes are involved, this implies in each case the intermediacy of molecules in which only one C-C bond has been formed. TWO pericyclic transition states are thus involved in this reaction. Molecular modelling implies the [#map first bond formation has a much higher energy then the subsequent second one] (by about 78 kJ/mol). Click on the diagram above to see models generated using molecular modelling. If instead, the [16]annulene is considered as a bond valence isomer (Note, this is NOT the same as a resonance isomer), then it adopts an entirely different shape from the other isomer. In fact, the first isomer has a plane of symmetry and the second isomer an exis of symmetry, but also a higher energy. With this new shape an alternative way of forming the two bonds can be drawn, which involves pushing one set of four arrows, and thus constitutes a single pericyclic 12 electron (4n) process formally equivalent to a cycloaddition reaction, but with the selection rules predicting a quite different stereochemical outcome! Here, the predictions of theory can be tested by observing the stereochemistry of the final product. [Very recently, another, lower energy, [#map isomer] with an axis of symmetry has been located]. A brain teaser is how to consider the analogous reaction of the [14]annulene, where both the two separate electrocyclic reactions AND the concurrent cycloaddition reaction actually now predict the same stereochemical outcome! - Carbanions contribute 2 electrons to the total count;
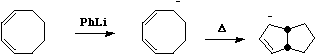
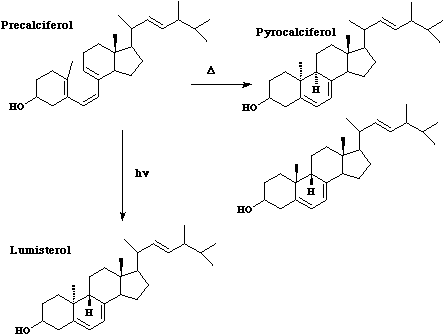
- Also in this category are some examples of steroidal reactions which could only be rationalised on the basis of the selection rules;
- Finally, as a conundrum, think about the following ring openings, and whether they proceed via a Huckel or a Mobius transition state?
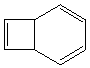
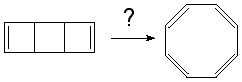 Is this last example an electrocyclic reaction or a cycloelimination reaction?
Is this last example an electrocyclic reaction or a cycloelimination reaction?
Examples Of Cycloaddition/Elimination Reactions.
- The simplest example is the well [DA.pdf known Diels Alder cycloaddition]. Two systems are involved, one contributing 4π electrons, the other 2π electrons. The total count is 6 (4n+2, n=1) and since the reaction is thermal, it must proceed via Huckel topology involving suprafacial components. These are considered as follows. Each π system forms two new σ bonds. These two new bonds can either form on the SAME face of oneπ system (suprafacial) or from opposite faces of one πsystem (antarafacial). A system of nomenclature has been developed to describe this. Hence the system below is classified as a π4s + π2s cycloaddition.
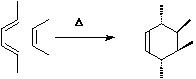 For a brief discussion of the application of frontier orbital method to cycloaddition reactions, [fmo.html see here].
For a brief discussion of the application of frontier orbital method to cycloaddition reactions, [fmo.html see here]. - An Example of an enzymically catalysed Diels-Alder reaction is the [#DAase unique enzyme extracted from Alternaria Solani] which catalyses a [2+4] cycloaddition in prosolanapyrone (III) with very high exo-selectivity (the normal selectivity is endo). For the structure of this enzyme, see [1934.pdf Science, 1998, 279, pp.1934-1940].
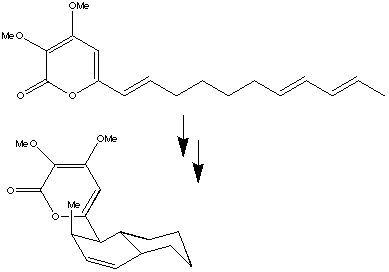 The stereochemistry defining the addition across the diene component. The stereochemistry defining the addition across the alkene component. Shown below is the alternative (endo) isomer. This too is suprafacial on both components, but the diene is attacked from the oppposite face to the exo isomer. Whilst either isomer is allowed by the pericyclic selection rules, the enzyme effectively selects only one of these, resulting from a "better fit" into the active site. This highly active area has been extensively reviewed: [DAase.pdf "Diels-Alderases"]; "[theozyme.pdf theozymes and compuzymes]" (theoretically constructed enzymes); [protein_cat.pdf protein-catalysed cycloaddition reactions] and [biosyn_da.pdf biosynthetic Diels-Alder] reactions
The stereochemistry defining the addition across the diene component. The stereochemistry defining the addition across the alkene component. Shown below is the alternative (endo) isomer. This too is suprafacial on both components, but the diene is attacked from the oppposite face to the exo isomer. Whilst either isomer is allowed by the pericyclic selection rules, the enzyme effectively selects only one of these, resulting from a "better fit" into the active site. This highly active area has been extensively reviewed: [DAase.pdf "Diels-Alderases"]; "[theozyme.pdf theozymes and compuzymes]" (theoretically constructed enzymes); [protein_cat.pdf protein-catalysed cycloaddition reactions] and [biosyn_da.pdf biosynthetic Diels-Alder] reactions - Diels Alder additions have also been observed catalysed within the [#DAase cavities of certain organic crystal structures]. In this example ([ja964198s.pdf J. Am. Chem. Soc., 1997, 119, 4117], Bull. Chem. Soc. Japan, 1997, 70, 3075-3079) one molecule of cyclohexadiene is encapsulated inside a cavity formed from four ligand molecules, along with TWO molecules of methyl methacrylate either side, one of which adds in Diels Alder fashion.
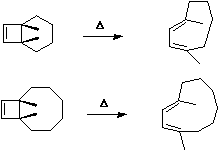
- This next example is a 4 electron (4n) cycloaddition reaction,
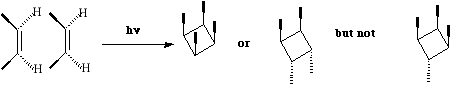 and photochemically proceeds via a Huckel topology (π2s + π2s). Thermally, the dimerisation of eg ethene is predicted by the selection rules to proceed as a π2s + π2a, the subscript a indicating an antarafacial component must be present on one alkene. However, there is a problem; an antarafacial mode implies a Mobius like twisting of 180° in the π system of the ethene, but ethene has only two carbons and cannot be twisted so much! Quantitative models indicate that only about 130° of twisting can actually be induced, of which about 80° occurs on one ethene and 50° occurs by twisting one ethene with respect to the other. Strain is also relieved by forming the two bonds at different rates.
and photochemically proceeds via a Huckel topology (π2s + π2s). Thermally, the dimerisation of eg ethene is predicted by the selection rules to proceed as a π2s + π2a, the subscript a indicating an antarafacial component must be present on one alkene. However, there is a problem; an antarafacial mode implies a Mobius like twisting of 180° in the π system of the ethene, but ethene has only two carbons and cannot be twisted so much! Quantitative models indicate that only about 130° of twisting can actually be induced, of which about 80° occurs on one ethene and 50° occurs by twisting one ethene with respect to the other. Strain is also relieved by forming the two bonds at different rates.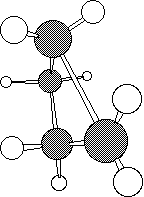
- The trans alkene shown below has sufficient driving force to actually indulge in this rather twisted transition state. Note that only the two strained trans double bonds react; the more stable cis double bonds do not participate in the reaction
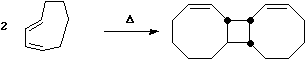
- A more common example of a presumed π2s + π2a reaction is the reaction between a ketene (or keteneimine) and alkene;
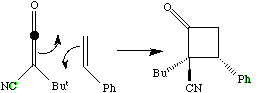 The antarafacial component is present only in the transition state, and it has the effect of giving the more sterically hindered product! It was noted above that one way of relieving strain induced by an antarafacial component is to make the two forming bonds of unequal length. In the extreme, for the ketene example above, this corresponds to; This of course is a stepwise rather than concerted reaction and is no longer a pericyclic process. Many 2+2 cycloadditions are thought to proceed in this manner, especially in more polar solvents which stabilise the intermediate zwitterions. In other systems, such asymmetry corresponds to the formation of so called "biradical intermediates", and recent evidence suggest these routes may be more common than hitherto suspected!
The antarafacial component is present only in the transition state, and it has the effect of giving the more sterically hindered product! It was noted above that one way of relieving strain induced by an antarafacial component is to make the two forming bonds of unequal length. In the extreme, for the ketene example above, this corresponds to; This of course is a stepwise rather than concerted reaction and is no longer a pericyclic process. Many 2+2 cycloadditions are thought to proceed in this manner, especially in more polar solvents which stabilise the intermediate zwitterions. In other systems, such asymmetry corresponds to the formation of so called "biradical intermediates", and recent evidence suggest these routes may be more common than hitherto suspected! 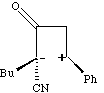
- In the next example, the reaction is classified as a π2s + π14a reaction, since 16 electrons = 4n (n=4) and hence again requires one antarafacial component.
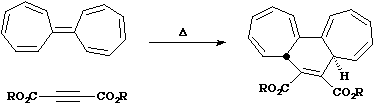 One point to note is that the antarafacial component could in principal occur on either alkene, but in practice the geometrical distortion is such that either the more strained or the larger component bears the antarafacial mode. As before, lone pairs count as two electrons, making the next example a six electron system; π2s + π4s
One point to note is that the antarafacial component could in principal occur on either alkene, but in practice the geometrical distortion is such that either the more strained or the larger component bears the antarafacial mode. As before, lone pairs count as two electrons, making the next example a six electron system; π2s + π4s 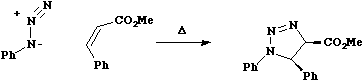
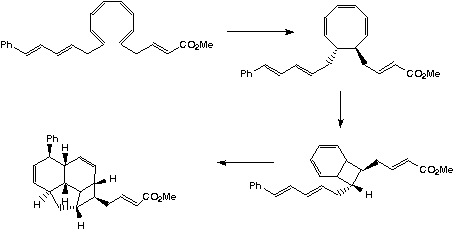
- The formation of [endiandric.mol endiandric acid] (Aust. J. Chem., 1982, 35, 557-565) involves the creation of eight chiral centres from a simple linear polyene precursor. This consists of a sequence of pericyclic reactions (a "pericyclic cascade or tandem reaction") to give the four membered ring system. Nicolaou ([ja00384a080.pdf J. Amer. Chem. Soc., 1982, 104, 5560-2]) unravelled the biomimetic pathway.
A subsequent intramolecular π2s + π4s Diels Alder cycloaddition gives the final product. The bonds formed in this sequence are- The first C-C bond formed by an electrocyclic reaction
- The second C-C bond form by an electrocyclic reaction
- The two C-C bonds form by a cycloaddition reaction
- The eight chiral centres are shown in yellow
Examples Of Sigmatropic Reactions.
- There are two different types of sigmatropic reaction, a) those that involve the migration of a hydrogen atom and b) those that involve a carbon or other atom. In the former category, the hydrogen atom can migrate either suprafacially or antarafacially across the conjugated system, leading to Huckel or Möbius topology for the transition states;
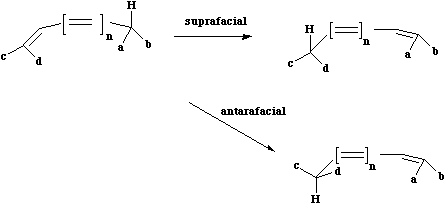
- Something different can occur when a carbon migrates.
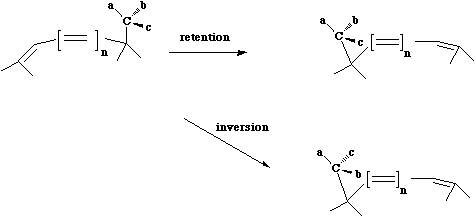
- The configuration at the migrating centre can be either retained (= suprafacial mode = Huckel topology) or inverted (= antarafacial mode = Möbius topology). Note that Möbius transition states are relatively common in this class because there is often little strain involved in inversion of configuration at a carbon. The most common category of hydrogen shift involves a so called [1,5] sigmatropic shift. As seen below this involves 6 electrons, and hence falls into the 4n+2 thermal category.
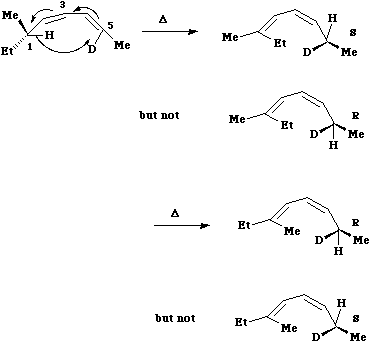 Here Huckel topology induces a suprafacial Hydrogen shift. The example shown involves a 'transfer of chirality' from the left hand side of the molecule to the right hand side. The absolute configuration of the two formed products shows unambiguously that the transfer of the hydrogen atom must occur suprafacially along the alkene.
Here Huckel topology induces a suprafacial Hydrogen shift. The example shown involves a 'transfer of chirality' from the left hand side of the molecule to the right hand side. The absolute configuration of the two formed products shows unambiguously that the transfer of the hydrogen atom must occur suprafacially along the alkene. - A practical example of this reaction involves the [ja992498e.pdf preparation of Chiral ethanoic (acetic) acid] (CHDTCO2H, where D= 2H and T=3H) which has long been an invaluable tool for elucidating biochemical mechanisms, but whose synthesis has been long, difficult and in low yield. Recently a new and particularly efficient route to this molecule has been developed, based on the reaction shown below. Heating the reactant gave a single product Z only if the alkyl group R = t-butyl. Smaller alkyl groups gave Z along with an isomer. Z was converted to chiral ethanoic acid (along with the three other products shown) by the sequence of three reagents shown. With R= t-Bu only a single product is formed, whereas with smaller R groups two compounds are formed.
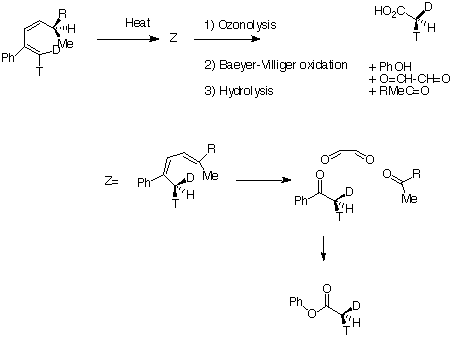 The reason for this can be seen more clearly when quantitative models of each transition state are constructed. The [#fav Favoured form] has a modelled energy of 63.7 kcal, whilst the [#unfav unfavoured form] has an energy of 69.7 kcal.
The reason for this can be seen more clearly when quantitative models of each transition state are constructed. The [#fav Favoured form] has a modelled energy of 63.7 kcal, whilst the [#unfav unfavoured form] has an energy of 69.7 kcal. - Another illustration of [1,5] hydrogen shifting can be [#a15 seen in cyclopentadiene]. The structure of this species implies that the 1H nmr spectrum should contain a triplet at about δ4 ppm corresponding to the sp3 protons and more peaks at about δ 5-6 corresponding to the sp2 protons, the ratio being 1:2.
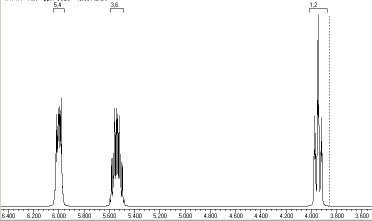 In fact, the room temperature spectrum shows only one peak at about 4.8 ppm. The reason for this is that a series of successive [1,5] hydrogen shifts is occurring so quickly that only the 'averaged' proton position is manifested in the nmr spectrum.
In fact, the room temperature spectrum shows only one peak at about 4.8 ppm. The reason for this is that a series of successive [1,5] hydrogen shifts is occurring so quickly that only the 'averaged' proton position is manifested in the nmr spectrum.
 Slowing down the exchange by cooling produces the expected spectrum described above! Many nmr spectra are affected by these so called 'degenerate' pericyclic processes, also called "ring whizzing".
Slowing down the exchange by cooling produces the expected spectrum described above! Many nmr spectra are affected by these so called 'degenerate' pericyclic processes, also called "ring whizzing". - The next example illustrates a [#a13 [1,3] migration.]
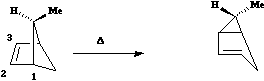 This involves 4 electrons, is hence a 4n process requiring Möbius topology, and this can be most easily achieved by inverting the configuration of the migrating carbon atom. If a model of this reaction is constructed, one can see that the configuration of the migrating sp3 carbon is indeed inverted.
This involves 4 electrons, is hence a 4n process requiring Möbius topology, and this can be most easily achieved by inverting the configuration of the migrating carbon atom. If a model of this reaction is constructed, one can see that the configuration of the migrating sp3 carbon is indeed inverted. - Next a cationic species, and from the numbering, it is classified as a [#a14 [1,4] carbon migration].
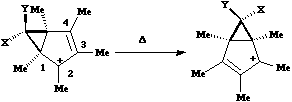 Again, the electron count corresponds to 4n, and again inversion at the migrating carbon atom is required. In this example this inversion is actually directly illustrated by inspecting the nmr spectrum. For the case where X and Y = Me, three and only three distinct methyl peaks are observed in the 1H nmr spectrum. This would only occur if [1,4] migration is fast, and also if inversion at the migrating centre were occurring, because this retains the individual identity of the two methyl groups X and Y. If retention at the migrating centre were to occur, X and Y would interchange their identity, and only two methyl peaks would have been observed. Note that if two more electrons were to be added to this system (ie the carbanion), the nmr spectrum would indeed be predicted to have only two methyl resonances.
Again, the electron count corresponds to 4n, and again inversion at the migrating carbon atom is required. In this example this inversion is actually directly illustrated by inspecting the nmr spectrum. For the case where X and Y = Me, three and only three distinct methyl peaks are observed in the 1H nmr spectrum. This would only occur if [1,4] migration is fast, and also if inversion at the migrating centre were occurring, because this retains the individual identity of the two methyl groups X and Y. If retention at the migrating centre were to occur, X and Y would interchange their identity, and only two methyl peaks would have been observed. Note that if two more electrons were to be added to this system (ie the carbanion), the nmr spectrum would indeed be predicted to have only two methyl resonances. - The next example is another cyclopropyl group "whizzing" around the ring. The CN group is always endo with respect to the large ring, from which one concludes that inversion at the migrating carbon must occur. Originally, it was thought this was an example of one 4n (n=2) [1,7] shift with one inversion, but a sequence of three alternative 4n (n=1) [1,3] shifts, with a cummulative single inversion can also provide a suitable pathway. Yet another possibility, that of [1,5] shifts with retention of configuration at the carbon, does not in fact provide a mechanism for "whizzing", since the cyclopropyl group cannot visit all the ring positions by this mechanism.
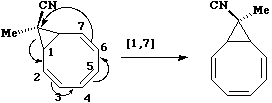
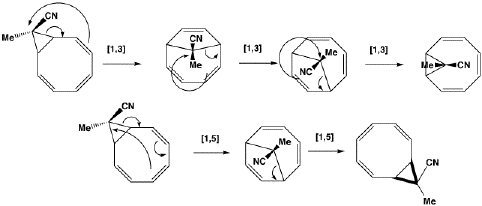
- This example is set up to show how a [1,5] migration of a methyl group can either go suprafacially with [#ss retention of configuration at the methyl (preserving a plane of symmetry)] or [#aa antarafacially with inversion of configuration at the methyl (preserving an exis of symmetry).] This last corresponds to two antarafacial components, but it is calculated to be about 23 kcal/mol (98 kJ/mol) higher in energy than the supra/supra mode. The larger ring provided by a [1,9] methyl shift does in fact allow the antara/inversion mode to energetically compete with the supra/retn mode; this experiment appears to have never been attempted!
- The next example is the one given in the introduction; the "Cope" rearrangement;
Here, an allyl group is migrating rather than a single carbon atom. The electron count is 6 (4n+2) and the reaction is thermally allowed via Huckel topology with suprafacial components, via a [#cope chair-like transition state]. When an oxygen atom is involved, the reaction is called a "Claisen rearrangement". - Another rare example of an enzymically catalysed sigmatropic reaction involves the Claisen type conversion of "[#chorismate chorismate]" to "[#chorismate prephenate]" by [#chorismate Chorismate mutase];
Much elegant work in this area was done by the Paul Bartlett group. . - When heteroatoms are involved, sigmatropic rearrangements involve odd numbers of atoms;
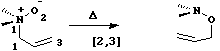
For this reaction too, an enzyme catalysed mode is known ([ja962257w.pdf An Antibody-Catalyzed [2,3]-Elimination Reaction], J. Amer. Chem. Soc., 1996, 118, 11686-11687). - Finally in this category, another conundrum. Can you classify the following reaction, and decide if it is thermally allowed or not?
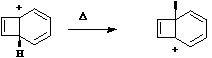
Return to Historical and Theoretical section.
The background to this lecture course can be found here.
--Rzepa 19:45, 12 April 2006 (bst)