Maariyah's Wiki Homepage:Suleman123
Task one
Optimised NH3 Molecule
Molecule name: Ammonia
H-N-H bond angle: 105.7
N-H bond length: 1.02 A
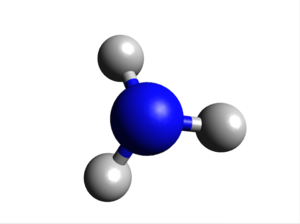
Calculation Method:
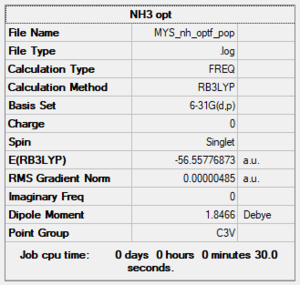
Item table:
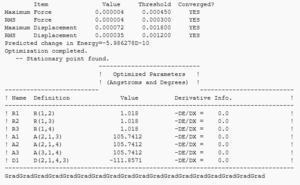
Vibrations:

Questions
1. Expected modes : 6
2. Degenerate modes : 2
3. Bending : H-N-H (1693.95-1) + N-H (1089.54cm-1)
4. Bond stretch : N-H symmetric (3461.29cm-1) + 2x asymmetric (3589.82-1)
6. Umbrella mode: N-H symmetric (3461.29cm-1)
7. Expected Experimental bands in NH3(g) : 4
Atomic charges:

XYZ File:
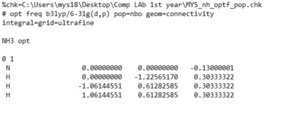
Jmol file:
JMol of ammonia |
Final optimisation file: log file
Optimised N2 Molecule
Molecule name: Nitrogen
N≡N bond length: 1.11 A
Calculation Method:

Item table:

Vibrations:
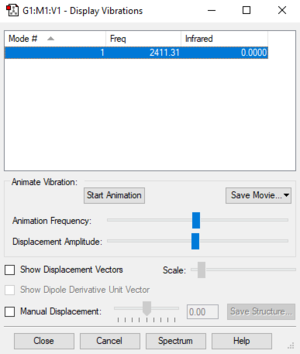
Atomic charges: Uncharged molecule, symmetrical.
Molecular Orbital:

Final optimisation file: Nitrogen.log
Optimised H2 Molecule
Molecule name: Hydrogen
H-H bond length: 0.74 A
Calculation Method:
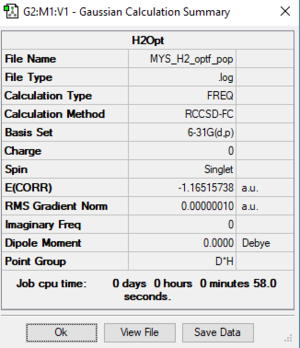
Item table:
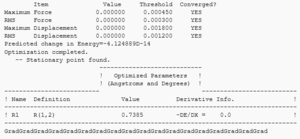
Vibrations:

Atomic charges: Uncharged molecule, symmetrical.
Molecular Orbital:
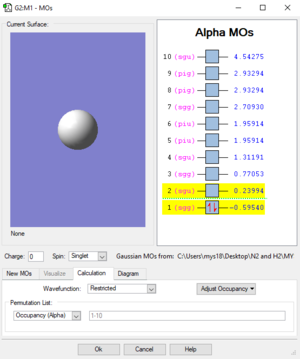
Final optimisation file: Hydrogen.log
Energy of Reaction
N2 + 3H2 → 2NH3
•E(NH3)= -56.55776873
•2*E(NH3)= -113.11553746
•E(N2)= -109.25579376
•E(H2)= -1.16515738
•3*E(H2)= -3.49547214
•ΔE = 2*E(NH3) - [E(N2 )+ 3*E(H2)] = -0.36427156 a.u
ΔE = -146.5 KJ/mol
Task two
Project molecule
Optimised CH4 Molecule
Molecule name: Methane
C-H bond length: 1.09 A
Point group: Td
