MRD:ql2817
This report has been plagiarised.
Question 1
On a potential energy surface diagram, how is the transition state mathematically defined? How can the transition state be identified, and how can it be distinguished from a local minimum of the potential energy surface?
The transition state is defined as the maximum on the minimum energy path linking reactants and the products.It is located at the saddle point in the potential energy surface diagram, which defined as : ∂V(ri)/∂ri=0 and H < 0, where H = frr(r0,V0)fVV(r0,V0)−f2rV(r0,V0) .
The transition state is the specific combination of internuclear distances AB and BC at which the trajectory is a point on the contour plot.
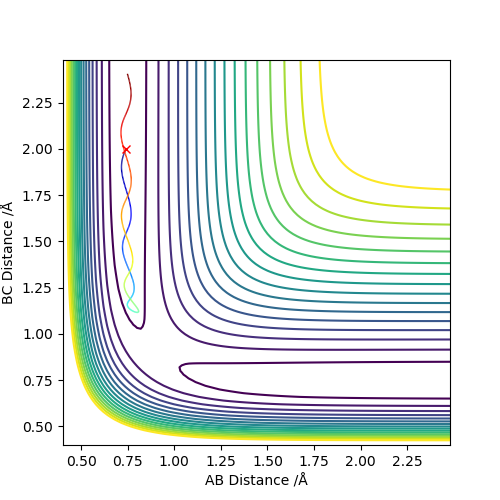
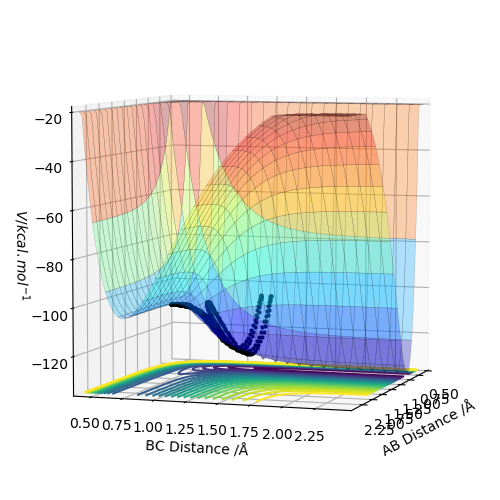
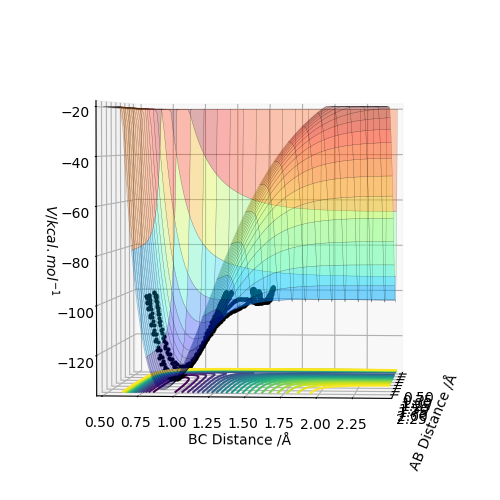
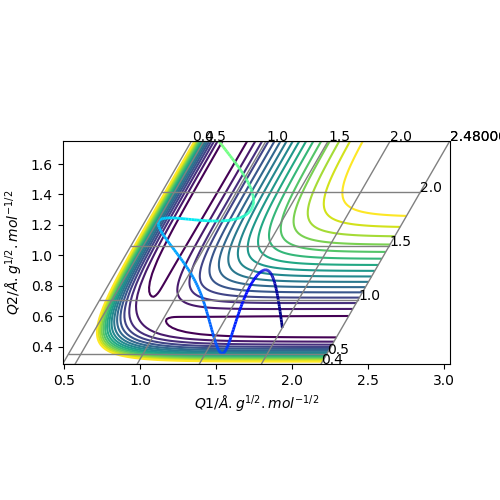
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” plot for a relevant trajectory.
When r(AB)=r(BC)=0.907 Å, p1 = p2 = 0.0, the transition state occurs. The internuclear distance vs time graph gives a relatively straight line which indicates a constant distance.
Comment on how the mep and the trajectory you just calculated differ.
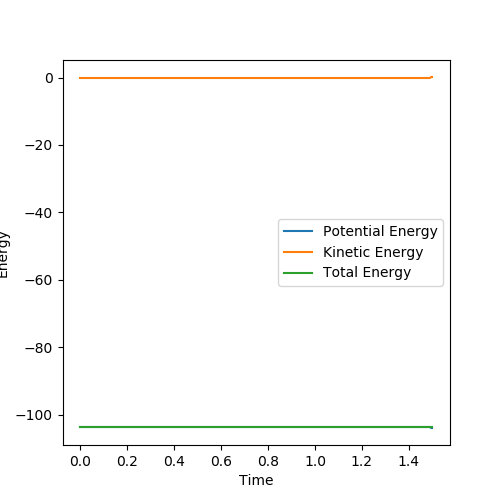
The mep trajectory keeps the potential energy at the minimum in a straight line, whereas the dynamic trajectory is wavy.
r1 = 0.907, r2 = 0.917
Molecules and atome moving away from each other.
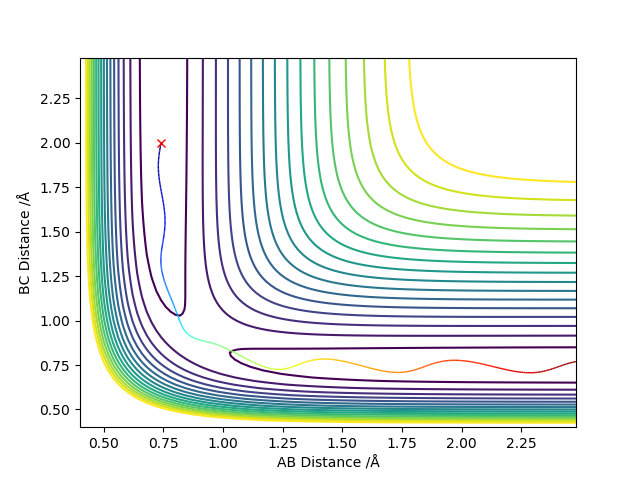
Setup a calculation where the initial positions correspond to the final positions of the trajectory you calculated above, the same final momenta values but with their signs reversed.
r1 = rts+δ, r2 = rts
final values of the positions r1(t) = 3.45, r2(t) = 0.72 and the average momenta p1(t) = -1.45, p2(t) = -2.48 at large t.
The molecule and the atom moving close together instead of moving apart.
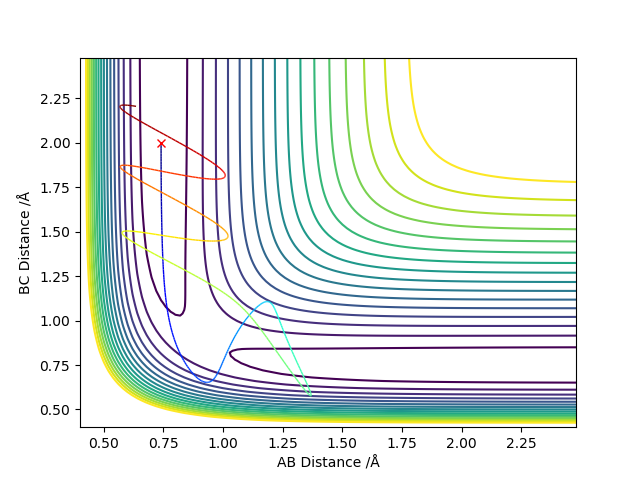
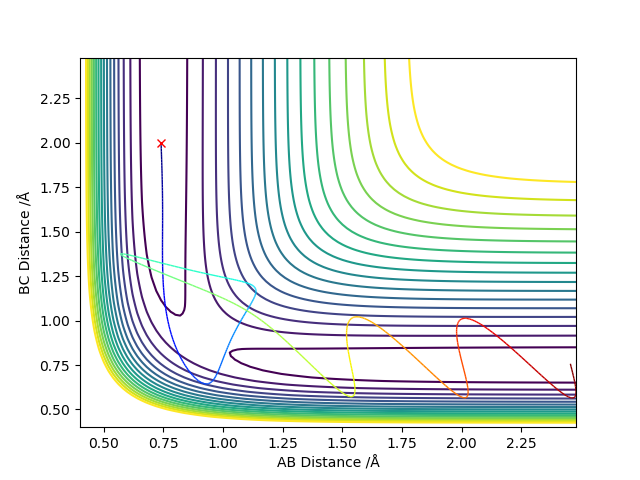
Complete the table above by adding the total energy, whether the trajectory is reactive or unreactive, and provide a plot of the trajectory and a small description for what happens along the trajectory. What can you conclude from the table?
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
Assumptions [1]
- Reactants are in constant equilibrium with the transition state structure.
- The energy of the particles follow a Boltzmann distribution.
- Once reactants become the transition state, the transition state structure does not collapse back to the reactants.
Transition state theory will predict a higher reaction rate than experimental one.
Exercise 2: F-H-H system
By inspecting the potential energy surfaces, classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved?
F+H2:
r(AB)=1.5,r(BC)=0.74, AB,BC m=0
exothermic, because the trajectory goes from a higher PE to a lower PE.
H+F2:
r(AB)=1.5,r(BC)=0.74, AB,BC m=0
endothermic, because the trajectory goes from a lower PE to a higher PE.
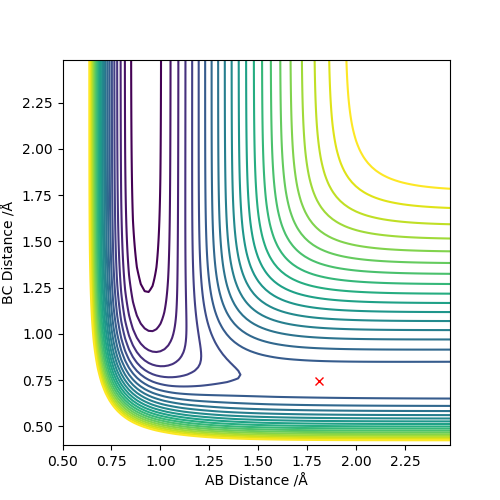
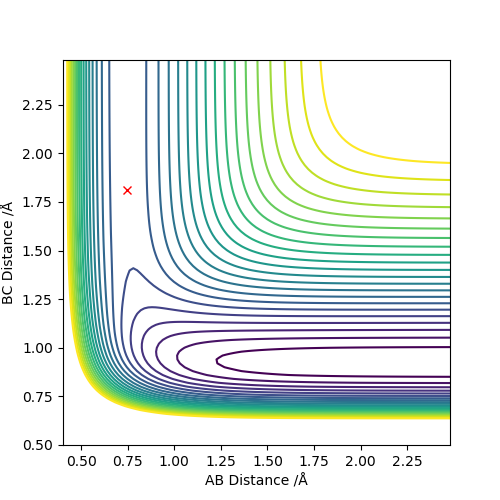
Locate the approximate position of the transition state.
F+H2:
r(AB): 1.8108 r(BC): 0.744877 AB BC m: 0
H+HF:
r(AB): 0.744877 r(BC): 1.8108 AB BC m: 0
Report the activation energy for both reactions.
F+H2:
activation energy: 30 kcal/mol
H+FH::
activation energy: 0.44kcal/mol
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. Explain how this could be confirmed experimentally.
reactive trajectory:
r(AB):1.6, r(BC):0.74, P AB:-0.5, P BC:0
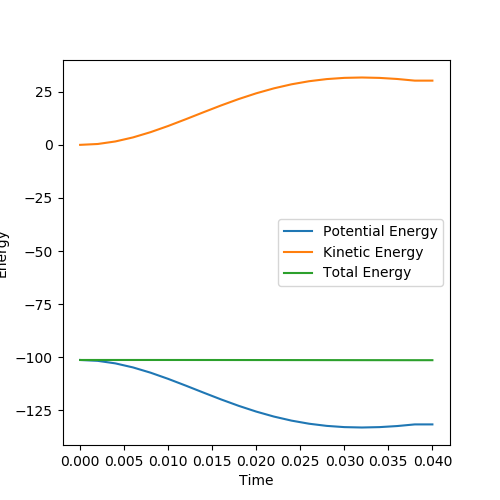
From the graph above, it is clearly shown that total energy remains the same. Kinetic energy increases to the same extent as potential energy decreases. This proves that energy is conserved. This can be confirmed experimentally by measuring the kinetic and potential energy in the real experiment, ie measure temperature change and potential energy can be measured using this software.
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state.
high translational energy, r(AB)=2, r(BC)=0.74, P AB=-7, P BC=0.5
high vibrational energy, r(AB)=2, r(BC)=0.74, P AB=-1, P BC=6
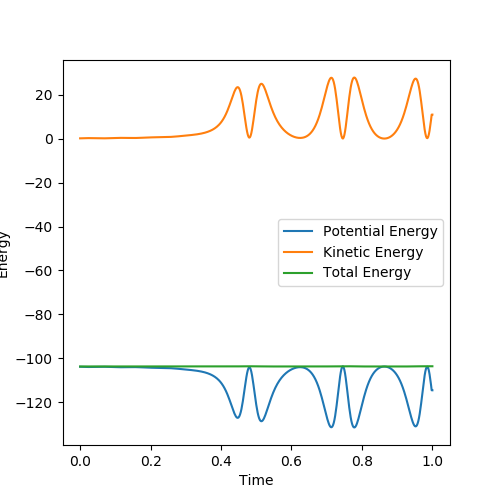
The reaction is a late TS reaction, also Polanyi's empirical rules tells us that vibrational energy is more important in compare to vibrational energy in a late transition state reaction, which can also be proved in the graphs above.
References
: