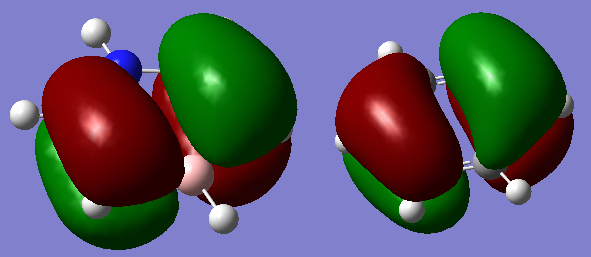MMOsm6416
BH3
B3LYP/6-31G(d,p)
Frequency file: BH3 log file
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000064 0.001800 YES RMS Displacement 0.000039 0.001200 YES
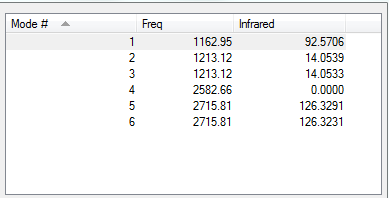
Low frequencies --- -14.5183 -14.5142 -10.8197 0.0008 0.0169 0.3454 Low frequencies --- 1162.9508 1213.1230 1213.1232
Optimised BH3 |
| Wavenumber (cm-1) | Symmetry | Intensity (arbitrary units) | IR active? | Type |
|---|---|---|---|---|
| 1163 | A2" | 92 | Yes | Out of plane bend |
| 1213 | E' | 14 | Slight | In plane bend |
| 1213 | E' | 14 | Slight | In plane bend |
| 2583 | A1' | 0 | No | Symmetric stretch |
| 2716 | E' | 126 | Yes | Asymmetric stretch |
| 2716 | E' | 126 | Yes | Asymmetric stretch |
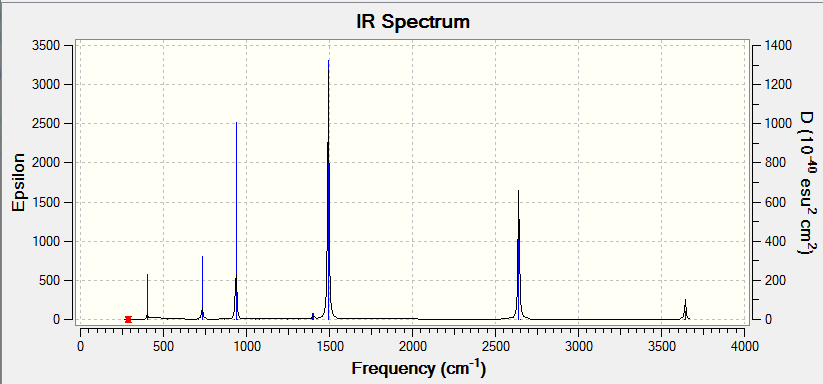
There are 3N-6 vibrations as expected of a non-linear molecule. However some vibrations are not IR active as they do not result in a change in dipole moment of borane, hence they are not seen in the spectrum. There are two sets of degenerate vibrations resulting in the same type of vibration but with different atoms involved.
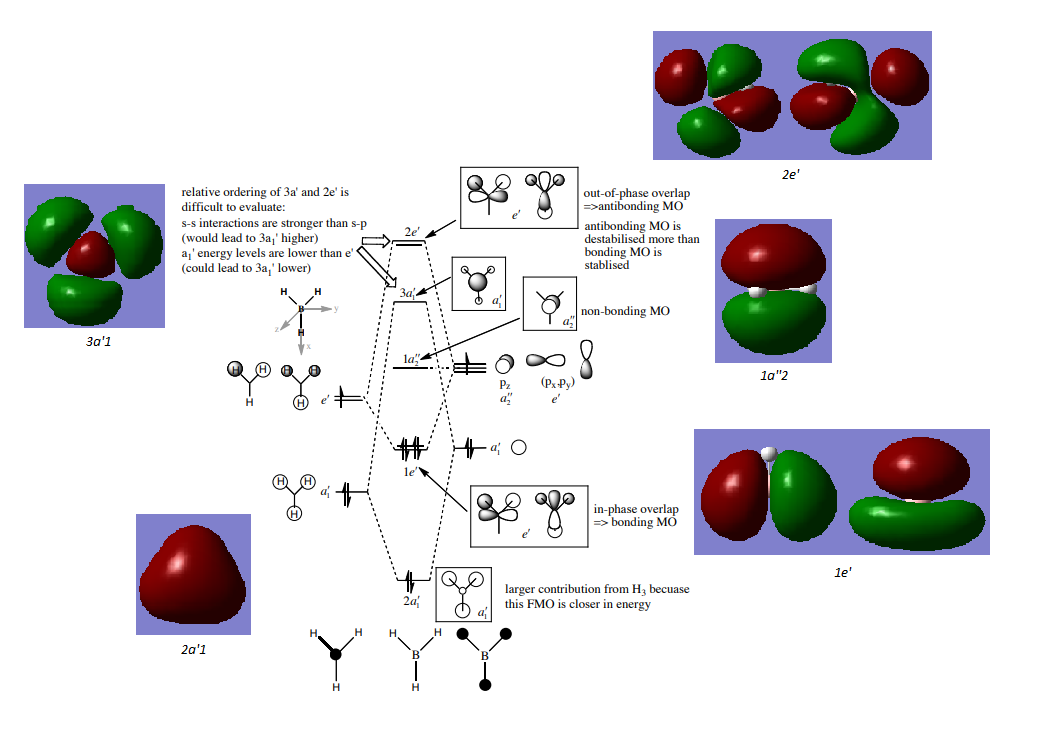
Diagram used from Dr. Patricia Hunt - Molecular Orbitals, Lecture 4 Tutorial Sheet - http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
Ng611 (talk) 22:05, 20 May 2018 (BST) Good analysis, well done!
There are some notable differences between the computed MOs and the LCAOs. The computational method directly solves the Schrodinger equation and is therefore more realistic of the true MOs in borane. The LCAO method is simple and quick to determine a rudimentary understanding of the bonding in a molecule, which is useful nevertheless. Take 2a1', the LCAO method does not indicate that there is bonding across the whole molecule, however the true computed MO highlights sigma bonding across the entirety of borane.
Ng611 (talk) 22:05, 20 May 2018 (BST) True, but a lack of nodal planes in the molecule should suggest to you that the electron density should be delocalized across the molecule. I would not say that this is a significant difference. A more significant one would be the size of the orbitals contributions.
Are there any significant differences between the real and LCAO MOs? What does this say about the accuracy and usefulness of qualitative MO theory?
NH3
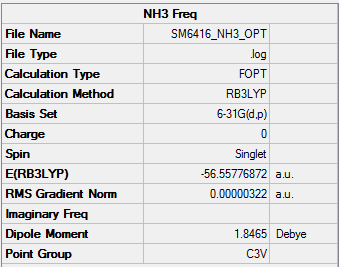
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000039 0.001800 YES RMS Displacement 0.000013 0.001200 YES
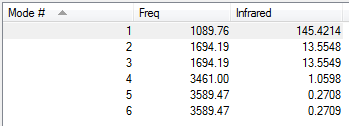
Low frequencies --- -8.5646 -8.5588 -0.0044 0.0454 0.1784 26.4183 Low frequencies --- 1089.7603 1694.1865 1694.1865
Frequency file: NH3 log file
Optimised NH3 |
| Wavenumber (cm-1) | Symmetry | Intensity (arbitrary units) | IR active? | Type |
|---|---|---|---|---|
| 1090 | A | 145 | Yes | Out of plane bend |
| 1694 | E | 14 | Slight | In plane bend |
| 1694 | E | 14 | Slight | In plane bend |
| 3461 | A | 1 | No | Symmetric stretch |
| 3589 | E | 0 | No | Asymmetric stretch |
| 3589 | E | 0 | No | Asymmetric stretch |
NH3BH3
B3LYP/6-31G(d,p)
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.000489 0.001800 YES RMS Displacement 0.000287 0.001200 YES
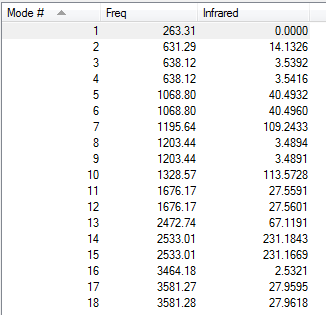
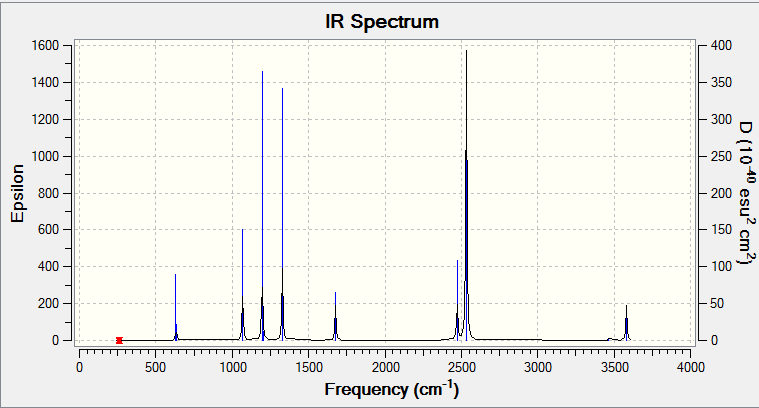
Low frequencies --- -16.7718 -0.2418 -0.0064 0.1514 15.7972 15.8690 Low frequencies --- 263.3158 631.2868 638.1190
Frequency file: NH3BH3 log file
Optimised NH3BH3 |
| Wavenumber (cm-1) | Symmetry | Intensity (arbitrary units) | IR active? | Type |
|---|---|---|---|---|
| 263 | A | 0 | No | N-B Twist |
| 631 | A | 14 | Slight | N-B Symmetric stretch |
| 638 | E | 4 | No | N-B Bend |
| 638 | E | 4 | No | N-B Bend |
| 1069 | E | 40 | Yes | N-B Bend |
| 1069 | E | 40 | Yes | N-B Bend |
| 1196 | A | 109 | Yes | BH3 Out of plane bend |
| 1203 | E | 3 | No | BH3 In plane plane |
| 1203 | E | 3 | No | BH3 In plane bend |
| 1329 | A | 114 | Yes | NH3 Out of plane bend |
| 1676 | E | 28 | Yes | NH3 In plane bend |
| 1676 | E | 28 | Yes | NH3 In plane bend |
| 2473 | A | 67 | Yes | BH3 Symmetric stretch |
| 2533 | E | 231 | Yes | BH3 Asymmetric stretch |
| 2533 | E | 231 | Yes | BH3 Asymmetric stretch |
| 3464 | A | 3 | No | NH3 Symmetric stretch |
| 3581 | E | 28 | Slight | NH3 Asymmetric stretch |
| 3581 | E | 28 | Slight | NH3 Asymmetric stretch |
Association Energy
| Molecule | Energy (a.u.) |
|---|---|
| NH3 | -56.55777 |
| BH3 | -26.61532 |
| NH3BH3 | -83.22469 |
ΔE = -0.0516 a.u. = -135 kJ/mol
Based on this information the B-N dative bond is weak and more ionic in character as compared to a C-C bond, common across all of Chemistry, with E = -350 kJ/mol, as the lone pair of electrons from N are being attracted into the empty p-orbital of B. Nevertheless, the bond is neither fully covalent nor fully ionic and lies inbetween the two.
Ng611 (talk) 22:06, 20 May 2018 (BST) Remember to include a comparative bond value and cite it (ideally from a textbook, databook, or paper).
BBr3
B3LYP/LanL2DZ
Frequency file: BBr3 log file
Item Value Threshold Converged? Maximum Force 0.000026 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000111 0.001800 YES RMS Displacement 0.000060 0.001200 YES
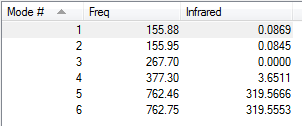
Low frequencies --- -3.7230 -2.7934 -2.0887 -0.0002 0.0001 0.0001 Low frequencies --- 155.8777 155.9513 267.7021
Optimised BBr3 |
| Wavenumber (cm-1) | Symmetry | Intensity (arbitrary units) | IR active? | Type |
|---|---|---|---|---|
| 156 | A' | 0 | No | In plane bend |
| 156 | A' | 0 | No | In plane bend |
| 268 | A' | 0 | No | Symmetric stretch |
| 377 | A" | 4 | No | Out of plane bend |
| 762 | A' | 320 | Yes | Asymmetric stretch |
| 763 | A' | 320 | Yes | Asymmetric stretch |
Aromaticity Project
Benzene
B3LYP/6-31G(d,p)
Frequency file: Benzene log file
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000082 0.000300 YES Maximum Displacement 0.000869 0.001800 YES RMS Displacement 0.000313 0.001200 YES
Low frequencies --- -11.6684 -0.0004 0.0004 0.0004 6.6654 15.6823 Low frequencies --- 414.0393 414.6034 621.0859
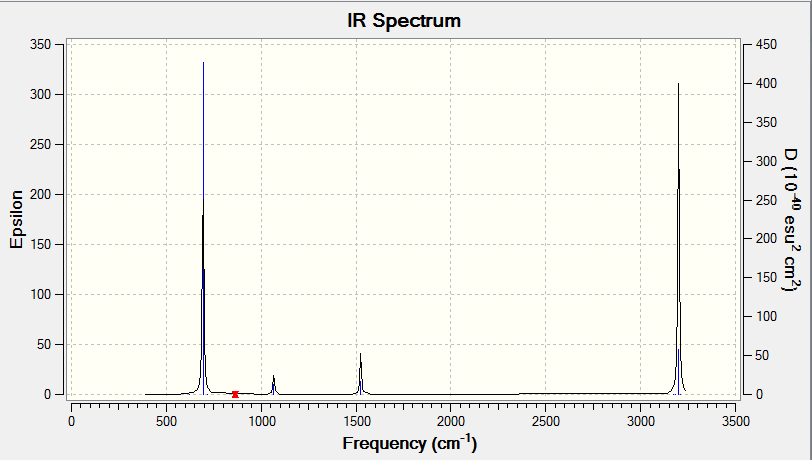
Optimised Benzene |
| Wavenumber (cm-1) | Symmetry | Intensity (arbitrary units) | IR active? | Type |
|---|---|---|---|---|
| 414 | A | 0 | No | Out of plane bend |
| 415 | A | 0 | No | Out of plane bend |
| 621 | A | 0 | No | In plane bend |
| 621 | A | 0 | No | In plane bend |
| 694 | A | 74 | Yes | Out of plane bend |
| 718 | A | 0 | No | Out of plane bend |
| 864 | A | 0 | No | Out of plane bend |
| 864 | A | 0 | No | Out of plane bend |
| 974 | A | 0 | No | Out of plane bend |
| 974 | A | 0 | No | Out of plane bend |
| 1013 | A | 0 | No | Out of plane bend |
| 1018 | A | 0 | No | In plane bend |
| 1020 | A | 0 | No | Breathing |
| 1066 | A | 3 | Slight | In plane twist |
| 1067 | A | 3 | Slight | In plane twist |
| 1179 | A | 0 | No | In plane bend |
| 1202 | A | 0 | No | In plane bend |
| 1202 | A | 0 | No | In plane bend |
| 1356 | A | 0 | No | C-C Asymmetric stretch |
| 1380 | A | 0 | No | In plane twist |
| 1524 | A | 7 | Slight | In plane twist |
| 1525 | A | 7 | Slight | In plane twist |
| 1653 | A | 0 | No | C-C Asymmetric stretch |
| 1653 | A | 0 | No | C-C Asymmetric stretch |
| 3175 | A | 0 | No | C-H Asymmetric stretch |
| 3184 | A | 0 | No | C-H Asymmetric stretch |
| 3184 | A | 0 | No | C-H Asymmetric stretch |
| 3200 | A | 47 | Yes | C-H Asymmetric stretch |
| 3200 | A | 47 | Yes | C-H Asymmetric stretch |
| 3210 | A | 0 | No | Breathing |
Borazine
B3LYP/6-31G(d,p)
Frequency file: Borazine log file
Item Value Threshold Converged? Maximum Force 0.000090 0.000450 YES RMS Force 0.000040 0.000300 YES Maximum Displacement 0.000990 0.001800 YES RMS Displacement 0.000292 0.001200 YES
Low frequencies --- -3.3677 -0.0009 0.0003 0.0009 8.8679 17.0303 Low frequencies --- 289.1601 289.6847 404.4233
Optimised Borazine |
| Wavenumber (cm-1) | Symmetry | Intensity (arbitrary units) | IR active? | Type |
|---|---|---|---|---|
| 289 | A | 0 | No | Out of plane bend |
| 290 | A | 0 | No | Out of plane bend |
| 404 | A | 23 | No | Out of plane bend |
| 525 | A | 1 | No | In plane bend |
| 525 | A | 1 | No | In plane bend |
| 710 | A | 0 | No | Out of plane bend |
| 712 | A | 0 | No | Out of plane bend |
| 733 | A | 60 | Yes | Out of plane bend |
| 864 | A | 0 | No | Breathing |
| 928 | A | 0 | No | Out of plane bend |
| 928 | A | 0 | No | Out of plane bend |
| 937 | A | 236 | Yes | Out of plane bend |
| 945 | A | 0 | No | In plane twist |
| 945 | A | 0 | No | Breathing |
| 945 | A | 3 | No | Breathing |
| 1052 | A | 0 | No | In plane bend |
| 1081 | A | 0 | No | In plane bend |
| 1081 | A | 0 | No | In plane bend |
| 1246 | A | 0 | No | In plane twist |
| 1314 | A | 0 | No | In plane twist |
| 1400 | A | 11 | Slight | In plane twist |
| 1401 | A | 11 | Slight | In plane twist |
| 1492 | A | 494 | Yes | In plane asymmetric stretch |
| 1493 | A | 494 | Yes | In plane asymmetric stretch |
| 2640 | A | 284 | Yes | B-H Asymmetric stretch |
| 2640 | A | 284 | Yes | B-H Asymmetric stretch |
| 2650 | A | 0 | No | B-H Symmetric stretch |
| 3642 | A | 0 | No | N-H Symmetric stretch |
| 3643 | A | 39 | Yes | N-H Asymmetric stretch |
| 3643 | A | 40 | Yes | N-H Asymmetric stretch |
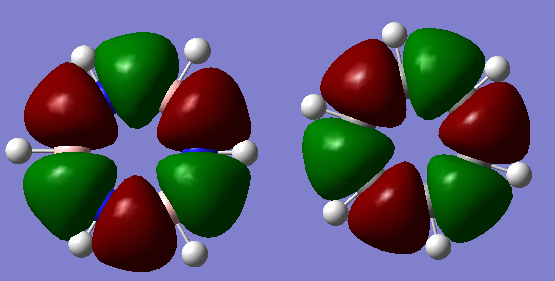
Charge Distribution
| Charge Comparison (Borazine vs Benzene) |
|---|
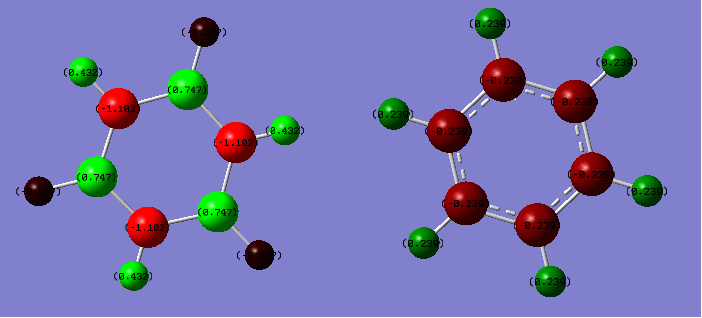
|
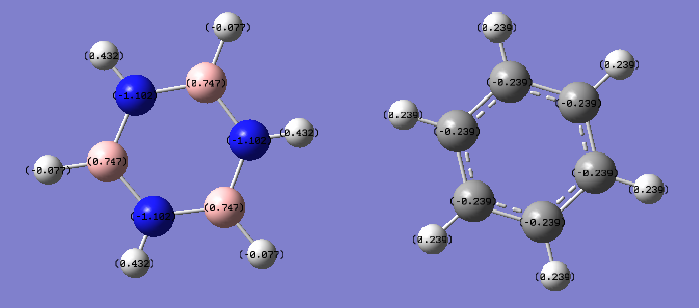
|
The NBO method to compare charge is much more effective than the Mulliken electronegativity method as it truly reflects the distribution of electrons across the whole molecule due to the MOs formed, thus treating the molecule as a whole and not considering individual bonds. Benzene has no dipole moment due to the pi ring formed of homonuclear carbons, where greater electron distribution resides in comparison to the hydrogens, as can be seen from the diagram. On the other hand, borazine has two different atoms forming the inner pi ring, namely boron and nitrogen. Borazine does have a dipole moment as a result of electron density being distributed unevenly across the molecule. This is due in part to the electronegativity of N being greater than that of B, thus electrons are more naturally localised around nitrogen. This goes further to show that B has a positive charge distribution associated, due to being adjacent to N. As a result of this positive charge on B, its adjacent hydrogen assumes a slightly negative charge distribution. In all the outside of borazine has hydrogens alternating between positive and negative charge.
Ng611 (talk) 22:09, 20 May 2018 (BST) Good discussion of the electronegativity of the atoms and reference to the electronegativity values. To improve consider other points such as symmetry and the overall neutral charge of the molecules (although you made a good reference to the overall dipole moment, well done).
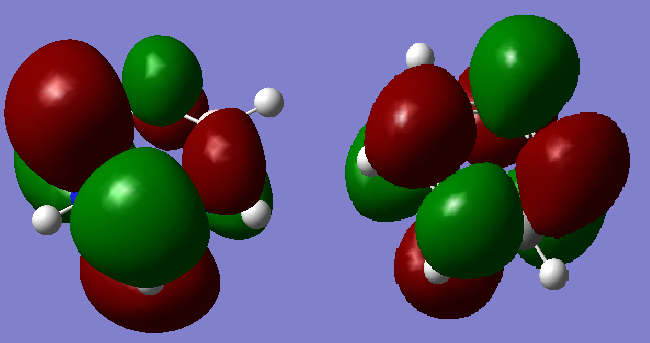
Molecular Orbital Comparison
Ng611 (talk) 22:13, 20 May 2018 (BST) Well done for comparing the correct MOs by shape and not energtic ordering (which is not necessarily reliable). I would include a brief discussion of the overall symmetry and electronegativity differences between the atoms in the molecule to improve this section further. Perhaps also consider dicussing the constituent AOs that form the MOs and the overall symmetry of the MO.
Aromaticity Summary
Aromaticity is the stabilisation exhibited by a planar ring as a result of electron delocalisation across the molecule. Computed MOs are a beneficial way to visualise the electron distribution across an aromatic molecule. Aromatic compounds obey the 4n+2 electron rule.
Further evidence for aromaticity derives in bond lengths. The distances between atoms in the ring reside between that of a single and double bond. A double bond signifies localised bonding, whereas with measured distances in aromatic compounds being between the two, this further shows the matter of electron delocalisation.
The extra stabilisation energy observed by aromatic compounds effectively 'protects' them against reactions such as hydrogenation, as it would be unfavourable for the molecule to lose this aromatic resonance energy. However, when considering a localised C=C double bond, these are readily hydrogenated due to the change in free energy being favourable.
It is understood that borazine exhibits aromatic behaviour like benzene due to the delocalised pi electron ring that exists perpendicular to the plane.
Pz orbitals existing in the plane are not elements of an aromatic compound, they are present as a form of a solid framework. It is the overlapping of of p orbitals perpendicular to the ring that result in electron density being delocalised across the whole molecule. LCAO provides a basic and quick understanding of this theory highlighting that delocalisation is the true reasoning behind aromatic resonance energies. Real MOs are very similar to their LCAO equivalent, however they often show electron density extending to regions in a molecule that cannot be predicted with the simple LCAO theory. It is in this manner that computed MOs are beneficial in the true understanding of chemical principles.