First steps with ONIOM
Create the first ONIOM molecule
Before going further, try to run our first ONIOM calculation !
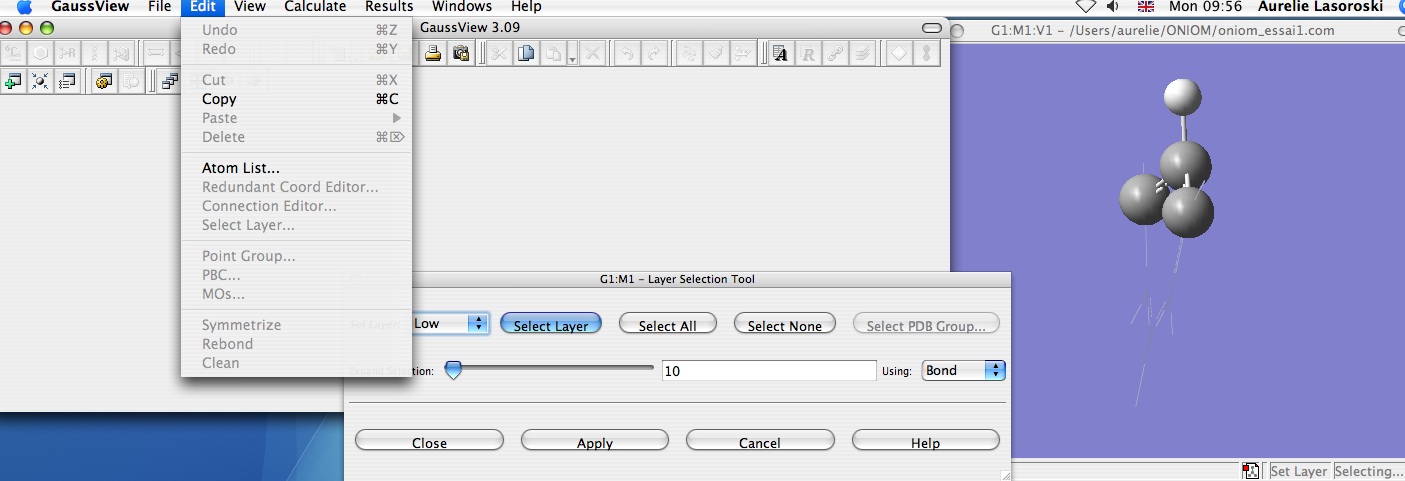
First of all, using GaussView, draw benzene and distort it, by moving a carbon out of the plane (this can be achieved by changing the dihedral angle). Then select the different areas of the molecule on which different levels of theory should be applied. (Edit --> Select Layer). For this ONIOM calculation the three carbons around the distorted centre have been selected as 'high level' and the three carbon atoms that remain in the plane have been selected as 'low level'.
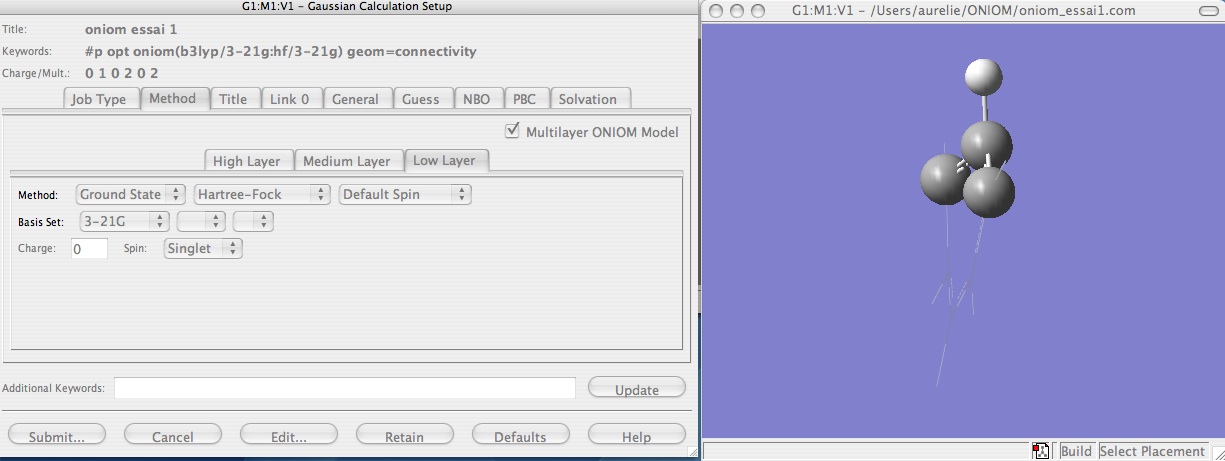
Finally, set up the ONIOM calculation (Calculate --> Gaussian Calculation Setup) by selecting the 'method' tab, and then choose the basis sets for the two different layers by selecting 'Multilayer ONIOM Model'. Here, B3LYP/3-21G has been selected for the high level theory, and HF/3-21G has been selected for the low level theory. Also remember to select optimization from the 'job type' tab.
Input
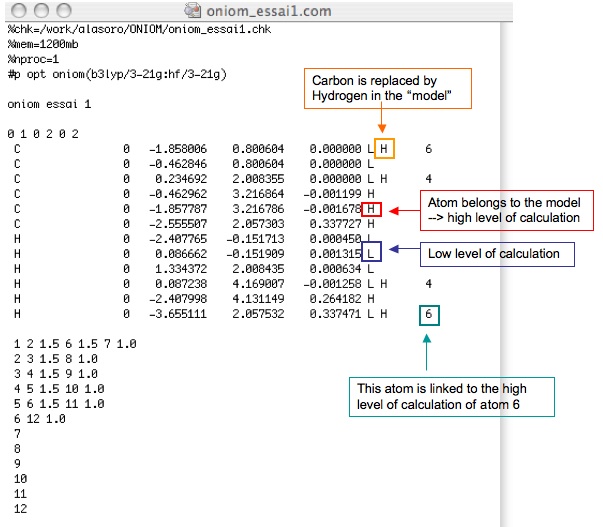
You must have an input like this one :
Output
In the output file the energy of the molecule at each iteration can be found. In fact there are four different energies listed, which correspond to terms in the following equation:
ONIOM: calculating energy. ONIOM: gridpoint 1 method: low system: model energy: -115.814040178590 ONIOM: gridpoint 2 method: high system: model energy: -116.609098705187 ONIOM: gridpoint 3 method: low system: real energy: -229.419112698610 ONIOM: extrapolated energy = -230.214171225207
So corresponds to ONIOM: extrapolated energy
Limitations
You have just completed your first ONIOM calculation, however, this is not a good example of what ONIOM can be used for. This is because the way that we delimited the system between the high and low level theories has broken the aromaticity of the benzene ring.
The primary use of ONIOM is to consider a specific region in which a chemical process occurs at a high level of theory, whilst using a low level of theory to model spectator regions of the system. This is because whilst the spectator regions interact with the region of interest, they do not require large amounts of computational time to provide an accurate model of the whole system, as they plays a more minor role in reactivity.
Another way to select layers
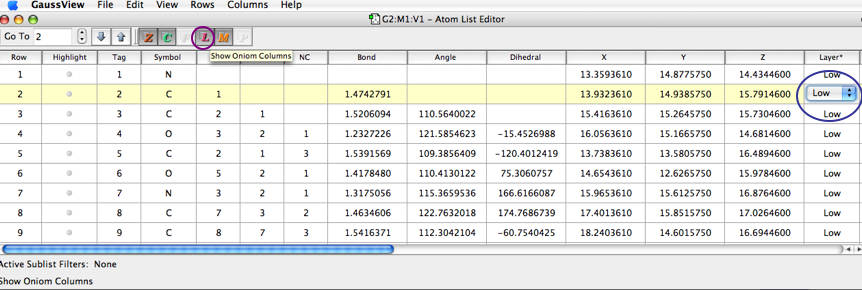
In order to select different layers you can also use the atoms list. I recommend this method when working with a big molecule (like a protein) in order to select a chromophore or specific amino acid to be calculated at a high level of theory.
Open the atom list (Edit --> Atom List) and then click on the 'L' button in the toolbar to show the ONIOM layer of each atom. Then click in the layer column and you will be able to change the layer of the atom.
Get back to Introduction
Next part ONIOM for excited states