Do NMR with Gaussian
Ab initio calculations can often be used to obtain reliable estimates of NMR chemical shifts, especially for organic compounds. In this part we will find some indications to use the NMR with Gaussian.
Calculations
In Calculate --> Gaussian --> Job style, choose NMR and select GIAO Method. Then click on Method and choose Hartree-Fock with a basis set of 3-21G or 6-21G (as usual).
Then click on Title, enter the title of your file, and click on Link 0. Type your file name after %chk. Then click on Edit and save on Gaussian. A third window should appear, in which you have to enter the file name, take the same as you enter after %chk (that is the last time that I make so much details) .
And naturally you can do the same with DFT
Obtain the NMR spectrum
Now you can obtain the NMR spectrum, you have to open the file .log with Gaussview (and no more with TextEdit). In this aim click on File --> Open, search for your file. Then when you will open the file a window should appear. It seems to be your molecule but in fact this molecule contain more informations as the last one because your NMR calculation can be extract from this one. So click on Results and now the NMR section is highlighted, click on.
Your spectrum should appear. In fact there is not just one spectrum but several because Gaussian made the calculations for all atoms. So in the case of the propene you can have H or C spectrum, you just have to choose what you want.
NMR spectrum of propene obtain with DFT (6-21G) (Hydrogene)
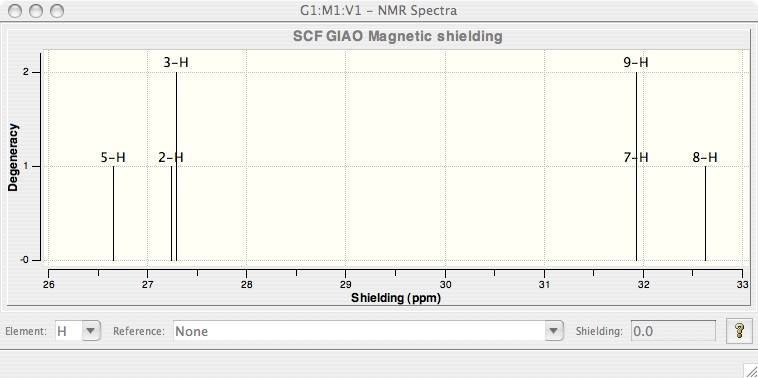
You can zoom on this spectrum, by selecting an area with the cursor, to be sure for example that two protons have exactly same shielding.
More explanations about NMR with Gaussian
Next part : Frequencies calculations
Back to Resgrp:comp-photo-c3h6-tutorial
Or go to : Some other methods : MPn, CCSD
The theoretical Background
Use Gaussian website : User's reference online
Unix reminder
