Zhexu616
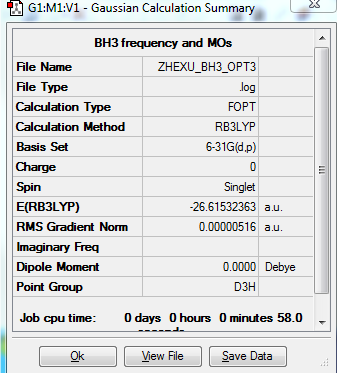
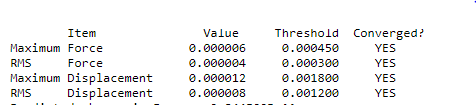
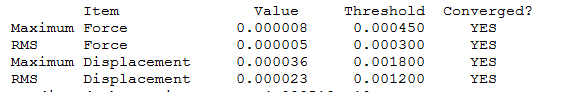
BH3
Optimization level used:B3LYP/6-31G level
The optimisation file is liked to here
Low frequencies --- -4.3230 -1.1744 -0.0054 1.1443 9.3738 9.4470
Low frequencies --- 1162.9805 1213.1720 1213.1746
BH3 |
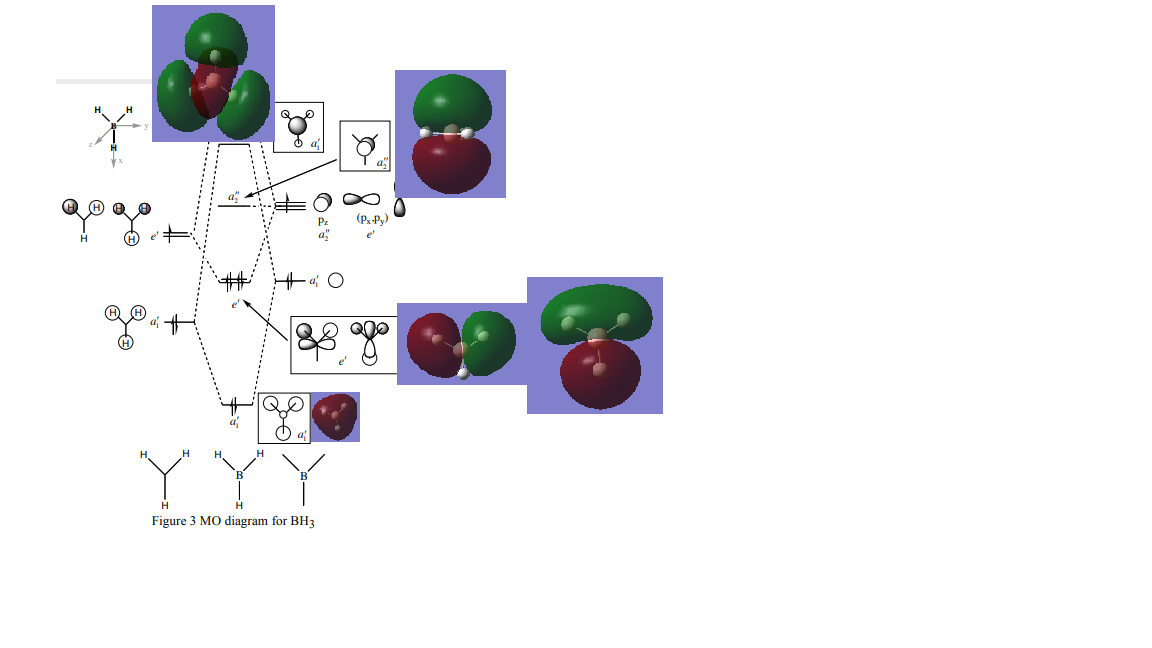
MO diagram from http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
Comments: There are differences between LCAO MOs and real MOs. Though LCAO MOs give a clear information about approximate electron distribution and the position of the nodal planes, real MOs provide straight foward 3D views. LCAO confined all electron densities within orbitals but real MOs depicted the real scenarios that electrons are everywhere in space. Real MOs are also good at providing information such as how diffuse a particular orbital is etc.. Qualitative MO theories are thus very useful.
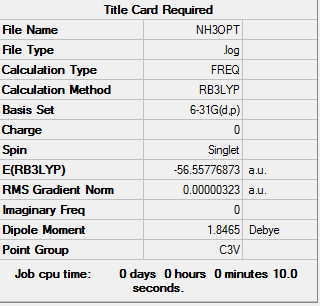
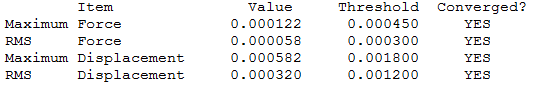
NH3
Optimization level used:B3LYP/6-31G level
the optimisation file can be found here
NH3 |
Low frequencies --- -0.0128 -0.0020 -0.0014 7.1034 8.1048 8.1051 Low frequencies --- 1089.3834 1693.9368 1693.9368
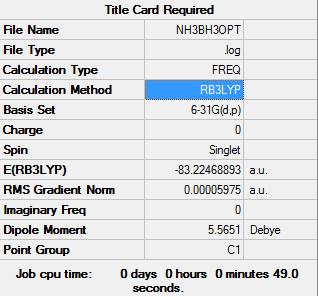
NH3BH3
Low frequencies --- -0.0006 0.0002 0.0008 16.8481 17.4133 37.2932
Low frequencies --- 265.8219 632.2116 639.3277
NH3BH3 |
Energy will be calculated by using the following equations:
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
E(NH3)=-56.55776873 a.u.
E(BH3)=-26.61532363 a.u.
E(NH3BH3)= -83.22468893 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]=-83.22468893 - (-26.61532363 - -56.55776873)=-113.16713403 a.u. = 2.97e5 kJ/mol
B-N bond is relatively weak, I compared the dissociation energy of BH3NH3 to B2H6
Ng611 (talk) 22:48, 15 May 2018 (BST) Correct values and correct formula applied but you swapped a plus sign for a minus sign! It should be -83.22468893 - (-26.61532363 + -56.55776873)
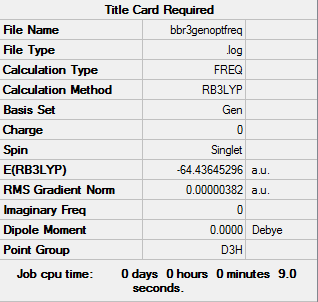
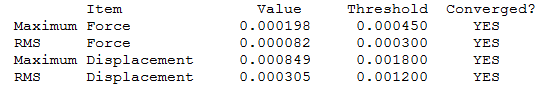
BBr3
Optimization level used:B3LYP/Gen level
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4311 2.4312 4.8420
Low frequencies --- 155.9631 155.9651 267.7052
I was not able to run the optimization from remote, therefore I ran it from local.
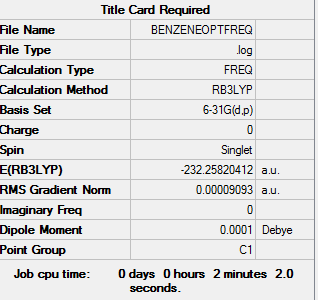
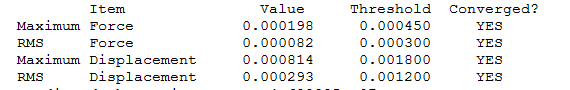
Benzene
Optimization level used:B3LYP/6-31G level
the optimisation file can be found here
benzene |
Low frequencies --- -13.7793 -12.9692 -11.9390 0.0002 0.0006 0.0006
Low frequencies --- 414.0698 414.1914 620.9704
Borazine
Optimization level used:B3LYP/6-31G level
the optimisation file can be found here
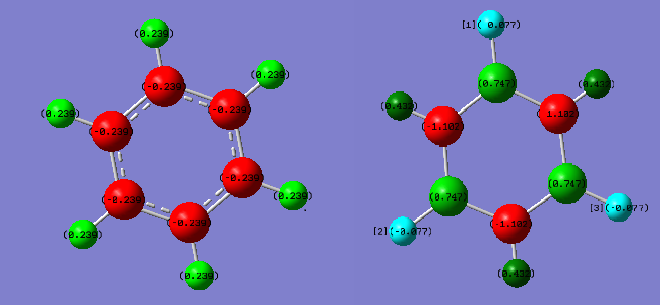
The graph shown above illustrated equal charges on hydrogen in benzene but two different charges in borazine, This is due to the difference of electronegativity between nitrogen and boron. Nitrogen has a higher electronegativity than boron, therefore the charge of hydrogen attached to nitrogen will be lower than that of boron. In addition, the electron deficient nature of boron compare to electron rich nitrogen also consolidate this behavior. The lone pair on nitrogen in sp3 orbital can donate into the ring therefore stabilise the molecule.
borazine |
Low frequencies --- -16.2225 -10.9996 -0.0003 0.0003 0.0007 1.9306
Low frequencies --- 288.8300 289.7582 404.2304
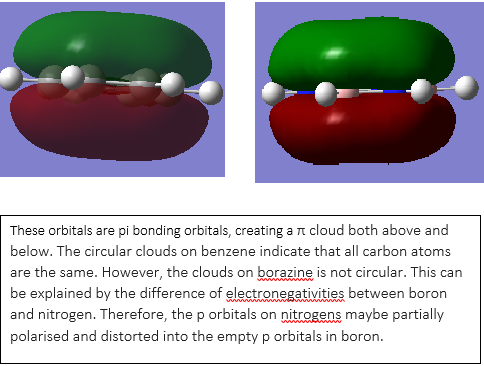
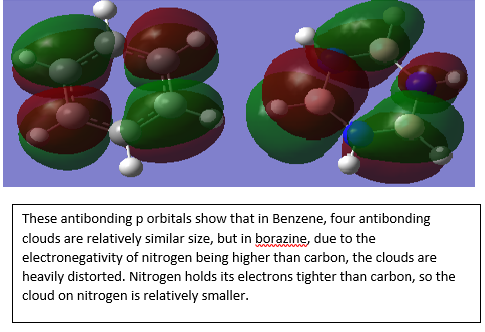
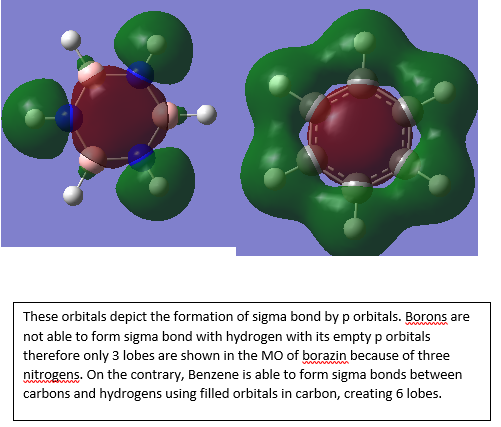
MO analysis
Ng611 (talk) 22:52, 15 May 2018 (BST) Good MO discussion, I'd include some additional information regarding the symmetry of the constituent AOs and the overall MO.
Aromaticity discussion:
Huckel's rule suggested that all planar cyclic molecules with 4n+2 pi electrons can be classified as aromatic, but this is only very limited. The real MOs provide better details in shapes as well as interactions between the orbitals. The sigma bond also contributes to the aromaticity in addition to delocalisation of pi electrons as seen in benzene's MOs. It is important that both global aromaticity or individual aromaticity depend heavily on electron density properties. MO theories are the best approach in analysing electron densities. However, MO theories have only limited explanation when it comes to bond lengths. When a hetroatom is present, Pz orbitals maybe polarised and distorted, however, this does not change the aromaticity of the molecule. There are also occasions especially when substrates are present that will alter the geometry of the Pz orbital or even interacting with Pz orbital without breaking the aromaticity. For a overall satisfactory understanding of aromaticity requires joint effort using multiple approaches but MO theories are the one which are most straight forward and comprehensible.
Ng611 (talk) 22:52, 15 May 2018 (BST) Good discussion! Don't be afraid to include more details in this discussion though, as it's a little too short.
Ng611 (talk) 22:53, 15 May 2018 (BST) A good overall report. Section 1 of this report was missing a number of items (e.g.: an incomplete MO analysis). You also carried out your association energy calculation incorrectly (you got the right equation and had the right numbers to plug in, but a silly error meant that you got the wrong number in the end). Section 2 was much better however, well done.