Zcj17y2comp
EX3 Section
BH3
B3LYP/6-61G level
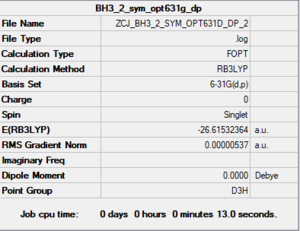
Item Table
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000019 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Frequency analysis log file:[[BH3|https://wiki.ch.ic.ac.uk/wiki/images/c/c2/ZCJ17_BH3_FREQ.LOG]]
Low Frequency Table
Low frequencies --- -7.5936 -1.5614 -0.0054 0.6514 6.9319 7.1055 Low frequencies --- 1162.9677 1213.1634 1213.1661
| Wavenumber (cm-1) | Intensity (arbitrary) | Symmetry | IR Active? | Type |
|---|---|---|---|---|
| 1163 | 92.6 | A2 | Yes | Out-of-plane bend |
| 1213 | 14.0 | E' | Very slight | In-plane bend |
| 1213 | 14.0 | E' | Very slight | In-plane bend |
| 2582 | 0 | A'1 | No | Totally symmetric stretch |
| 2716 | 126.3 | E' | Yes | Asymmetric stretch |
| 2716 | 126.3 | E' | Yes | Asymmetric stretch |
IR Spectrum
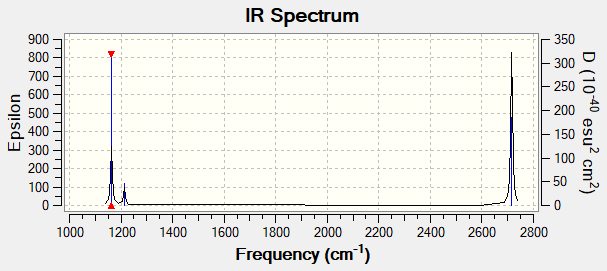
Ng611 (talk) 23:46, 21 May 2019 (BST) Why are there only three peaks in your IR spectrum?
JMol
optimised BH molecule |
BH3 MO Diagram
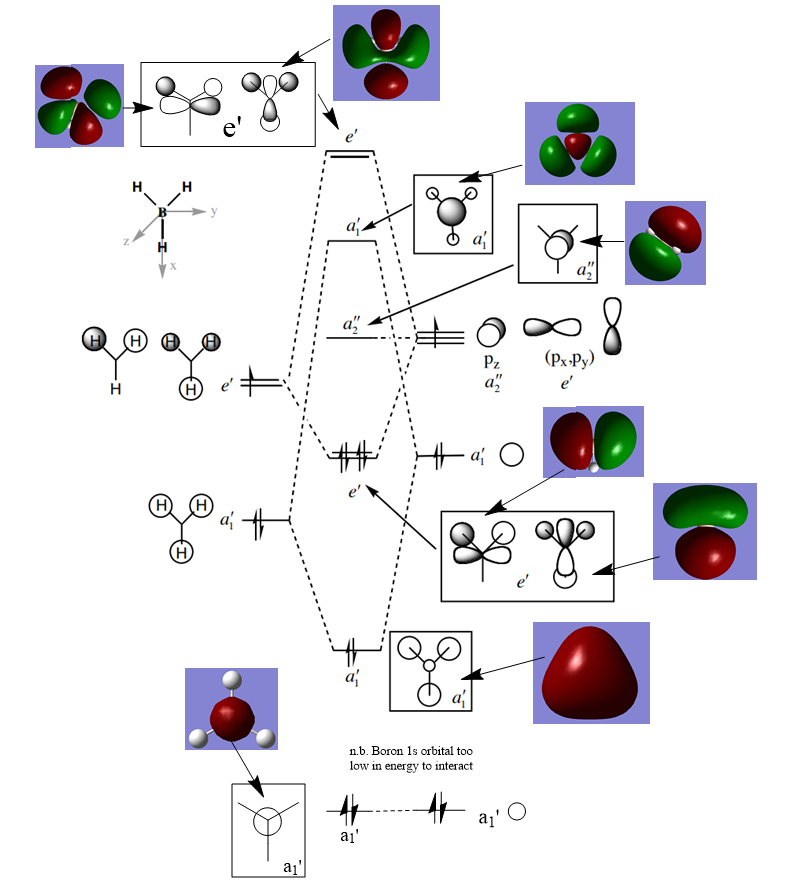
Diagram adapted from http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
Are there any significant differences between the real and LCAO MOs?
There are some significant differences between the MOs formed from the LCAO process and the computer-generated MOS. Firstly, The The LCAO MOs are simply superimposed atomic orbitals. This means it is not possible to see the total effect on placing two orbitals near each other. In contrast the 'real' MOS are created by fully calculating the effect of all of the different atomic or fragment orbitals involved. This means they appear more diffuse and, in some cases, less clear in precisely which orbitals are involved.
What does this say about the accuracy and usefulness of qualitative MO theory?
The LCAO MOs are more qualitative and therefore, are less accurate as a true picture of molecular orbitals as the computer generated MOs. This inherently makes them less useful in complex molecular analysis. However, they do have some use in identifying specifically which atomic orbitals are involved in certain bonding interactions.
Ng611 (talk) 23:52, 21 May 2019 (BST) Whilst MO theory doesn't predict the exact shape of the MOs, this isn't the most major difference. What about the relative contributions of the individual AOs?
NH3BH3
NH3
B3LYP/6-61G level
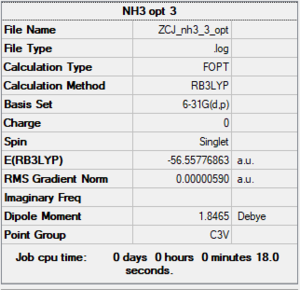
Item Table
Item Value Threshold Converged? Maximum Force 0.000009 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000019 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Frequency analysis log file:[[NH3|https://wiki.ch.ic.ac.uk/wiki/images/2/21/ZCJ_NH3_3_FREQ.LOG]]
Low Frequency Table
Low frequencies --- -8.5223 -8.4750 -0.0037 0.0334 0.1918 26.4067 Low frequencies --- 1089.7616 1694.1862 1694.1866
JMol
optimised NH molecule |
NH3BH3
B3LYP/6-61G level
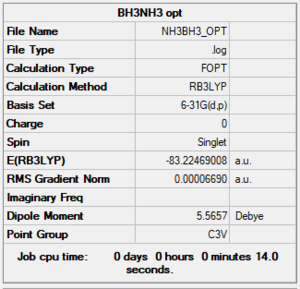
Item Table
Item Value Threshold Converged? Maximum Force 0.000139 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000771 0.001800 YES RMS Displacement 0.000338 0.001200 YES
Frequency analysis log file:[[NH3BH3|https://wiki.ch.ic.ac.uk/wiki/images/7/72/ZCJNH3BH3_FREQ.LOG]]
Low Frequency Table
Low frequencies --- -0.0614 -0.0448 -0.0066 22.0873 22.0930 40.5268 Low frequencies --- 265.8900 632.3722 640.1154
JMol
optimised BH molecule |
Energies
E(NH3)= -56.5578 a.u.
E(BH3)= -26.61532 a.u.
E(NH3BH3)= -83.2247 a.u.
Ng611 (talk) 23:58, 21 May 2019 (BST) You've rounded to early here. Your results should be reported to 5 d.p. and then your final answer is rounded to the nearest kJ/mol. Because you rounded to early, your answer is off by 1 kJ/mol.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)
=-83.22469 - [-56.55777 + -26.6153] = -0.05162 a.u. = -136 kJ/mol
The N-B bond energy is fairly weak. In comparison, a C-C single bond in ethene is 347 kJ/mol (from http://butane.chem.uiuc.edu/cyerkes/Chem104ACSpring2009/Genchemref/bondenergies.html%7Cdate accessed 10/05/19), which is about 2.5 times the strength.
Ng611 (talk) 23:58, 21 May 2019 (BST) A book source would be better here.
NI3
B3LYP/6-61G level

Item Table
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000022 0.001800 YES RMS Displacement 0.000014 0.001200 YES
Frequency analysis log file:[[NI3|https://wiki.ch.ic.ac.uk/wiki/images/e/e6/ZCJ_NI3_FREQ.LOG]]
Low Frequency Table
Low frequencies --- -12.5522 -12.5460 -6.0047 -0.0040 0.0191 0.0664 Low frequencies --- 100.9969 100.9977 147.3377
JMol
optimised BH molecule |
N-I optimised distance- 2.1840 Å
Project Section
N(CH3)4+
B3LYP/6-61G level
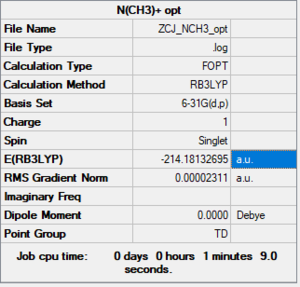
Item Table
Item Value Threshold Converged? Maximum Force 0.000073 0.000450 YES RMS Force 0.000018 0.000300 YES Maximum Displacement 0.000277 0.001800 YES RMS Displacement 0.000088 0.001200 YES
Frequency analysis log file:[[File:N(CH3)4+|https://wiki.ch.ic.ac.uk/wiki/images/7/79/ZCJ_NCH3_FREQ.LOG]]
Low Frequency Table
Low frequencies --- -0.0008 -0.0006 -0.0002 35.6259 35.6259 35.6259 Low frequencies --- 215.5175 315.1186 315.1186
JMol
optimised N(CH) molecule |
P(CH3)4+
B3LYP/6-61G level
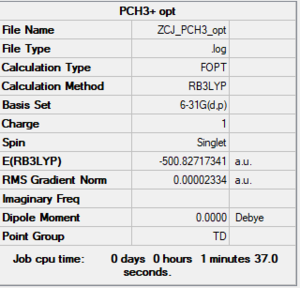
Item Table
Item Value Threshold Converged? Maximum Force 0.000138 0.000450 YES RMS Force 0.000035 0.000300 YES Maximum Displacement 0.000718 0.001800 YES RMS Displacement 0.000298 0.001200 YES
Frequency analysis log file:[[P(CH3)4+|https://wiki.ch.ic.ac.uk/wiki/images/d/d5/ZCJ_PCH3_FREQ.LOG]]
Low Frequency Table
Low frequencies --- -0.0024 -0.0023 -0.0016 51.6355 51.6355 51.6355 Low frequencies --- 188.7455 213.5999 213.5999
JMol
optimised P(CH) molecule |
Charges

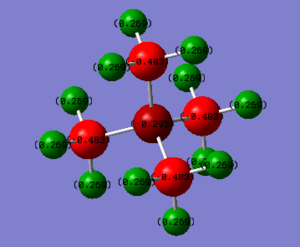
| Atom | Electronegativity (from http://www.rsc.org/periodic-table/trends) |
|---|---|
| Phosphorous | 2.19 |
| Nitrogen | 3.04 |
| Carbon | 2.55 |
| Atom | P(CH3)4+ | N(CH3)4+ | Notes |
|---|---|---|---|
| P/N | 1.666 | -0.295 | The phosphorous charge is positive, whereas the nitrogen's is negative. This is due to the difference in electronegativities. Nitrogen has an electronegativity of 3.04, whereas phosphorous has an electronegativity of 2.19. A low electronegativity means an atom can more easily stabilise a positive charge. In the nitrogen cation, the positive charge is shared between the nitrogen and the carbons. In the phosphorous cation, the charge is more localised to the phosophorous atom, however in all cases, charge is spread around the molecule to a certain extent |
| Carbon | -1.060 | -0.483 | |
| Hydrogen | 0.298 | 0.269 |
Ng611 (talk) 00:04, 22 May 2019 (BST) You should also discuss the charges on other atoms, as well as provide a discussion about differences between the formal charge picture and the calculated NBOs.
MOs
Ng611 (talk) 00:09, 22 May 2019 (BST) Good LCAO analysis! I'd label your interactions on the LCAO diagram instead of describing them in a paragraph under the diagrams.
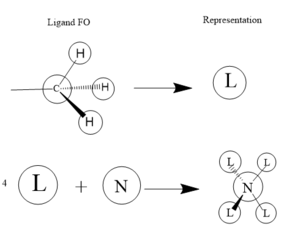

This MO is totally bonding. It is constructed from the 1s hydrogen orbitals and the 2s carbon and nitrogen oribitals all constructively overlapping
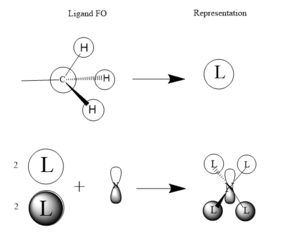
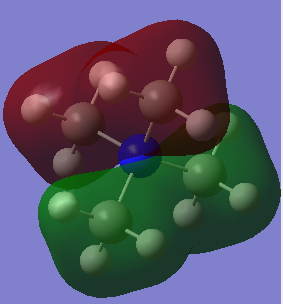
This MO is also totally bonding. It is constructed from the 1s hydrogen orgitals overlapping constructively with a carbon 2s orbital. These ligands then overlap constructively witht he nitrogen 2p orbitals, 2 as is, and 2 with inverted phase.
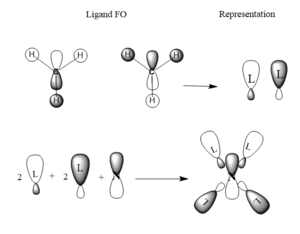
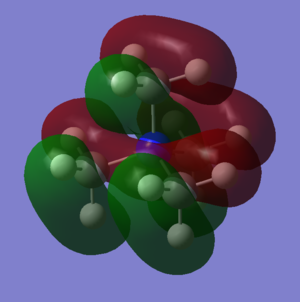
This is also a bonding orbital, with some antibonding charactetr through space. It is constructed from the hydrogen 1s orbitals overlapping in phase witht the carbon 2p orbital which is is then overlapping in phase again with the nitrogen 2p orbital for each ligand.
