ZQR01333824
Small molecules
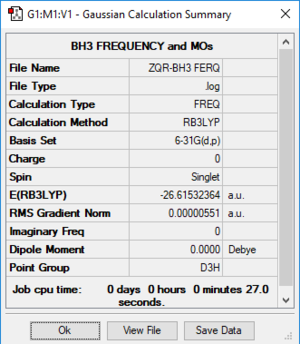
BH3 Molecule
Basic Information
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Summary table
Item table
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000043 0.001800 YES RMS Displacement 0.000022 0.001200 YES
Frequency log file
Low frequency table
Low frequencies --- -14.6858 -14.6818 -11.0436 -0.0009 0.0165 0.3415 Low frequencies --- 1162.9492 1213.1220 1213.1222
J mol file
BH Molecule |
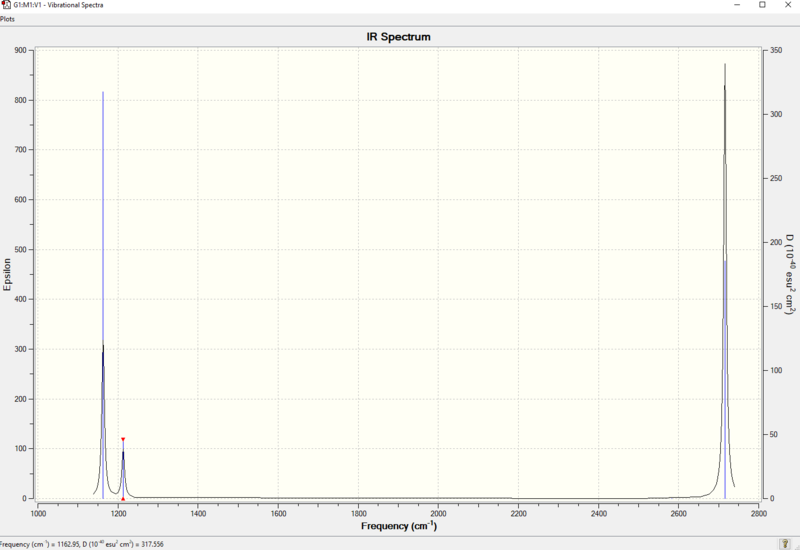
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 92 | A2 | Yes | out-of-plane bend |
| 1213 | 14 | E' | Yes | bend |
| 1213 | 14 | E' | Yes | bend |
| 2583 | 0 | A1 | no | symmetric stretch |
| 2716 | 126 | E' | Yes | asymmetric stretch |
| 2716 | 126 | E' | Yes | asymmetric stretch |
IR spectrum of BH3
Question
A: There are only three peaks on IR spectrum, which represents mode 1, mode 2 & 3 and mode 5 & 6. Mode 1 is IR active because there is change in dipole moment. Mode 2 and mode 3 are degenerate so they show as one peak. Same for mode 5 and 6 since they are also degenerate. Mode 4 is IR inactive because there is no net change in dipole moment.
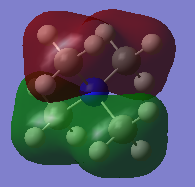
MO diagram of BH3
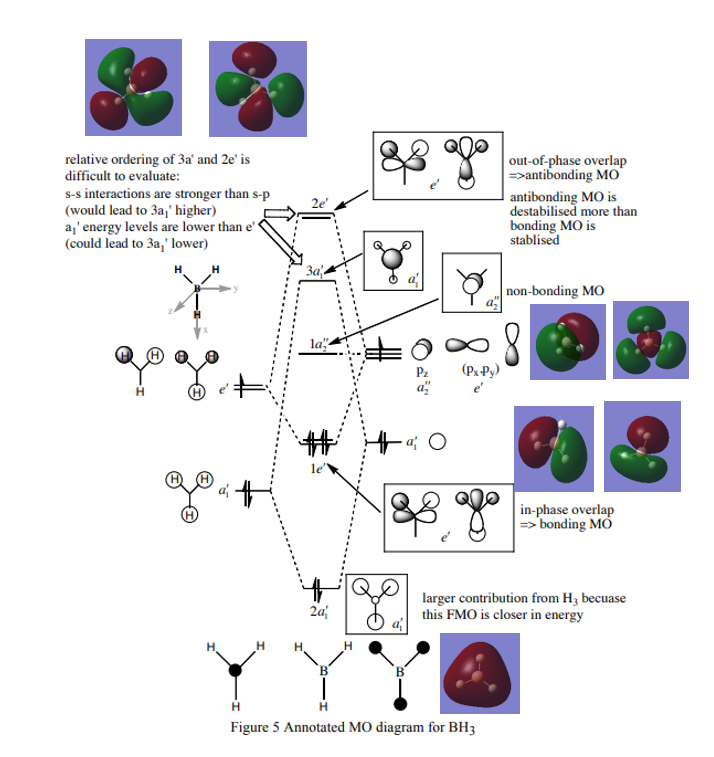
Reference: Hunt P. Lecture_4_Tut_MO_diagram_BH3. Inorganic Lecture Course; 2019.
Question
Q1 A: The real Mo is calculated by computer, it shows the real size, fragmentation of orbitals and the exact way they mix. LCAO is formed by combination of atomic orbitals, the exact size cannot be predicted accurately.
Q2 A: Qualitative Mos are only useful to predict the size and shape orbitals for small molecules, For more complex Mos, calculation is needed to get the accurate prediction.
Correct inclusion of the calculated MOs on to the MO diagram. Your evaluation of the usefulness of the LCAO approach is ok but to improve, you could have discussed the similarities and differences in more detail, for example, consider the differences in orbital contributions in the 3a1' or 2e' MOs. Smf115 (talk) 14:46, 2 June 2019 (BST)
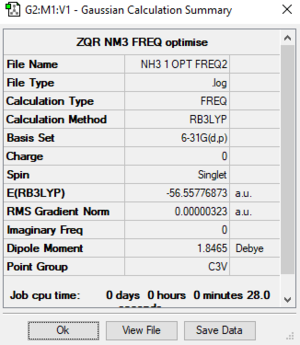
NH3 Molecule
Basic Information
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Summary table
Item table
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000013 0.001800 YES RMS Displacement 0.000007 0.001200 YES
Frequency log file
Low frequency table
Low frequencies --- -0.0129 -0.0018 0.0001 7.0724 8.1020 8.1023 Low frequencies --- 1089.3849 1693.9369 1693.9369
J mol file
NH Molecule |
NH3BH3 Molecule
Basic Information
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Summary table

Item table
Item Value Threshold Converged? Maximum Force 0.000350 0.000450 YES RMS Force 0.000112 0.000300 YES Maximum Displacement 0.001347 0.001800 YES RMS Displacement 0.000450 0.001200 YES
frequency log file
Low frequency table
Low frequencies --- -0.0265 -0.0066 -0.0053 10.4126 10.4592 38.3629 Low frequencies --- 265.3603 634.4262 639.2330
J mol file
BH Molecule |
Association energy
E of BH3 = -26.61532 a.u.
E of NH3 = -56.55777 a.u.
E of NH3BH3 = -83.22469 a.u.
ΔE= -83.22469-(-26.61532-56.55777) = -0.05160 a.u. = -135 kJ/mol
B-N dative bond is relatively weak compared to C-C bond which is equal to 346 KJ/mol
Correct calculation, good consideration given to the accuracy of the final reported energy values and ok comparison made. To improve, you could have considered why the C-C bond is a good comparison and always include a reference for literature values! Smf115 (talk) 14:48, 2 June 2019 (BST)
NI3 Molecule
Basic Information
Calculation method:RB3LYP
Basis set:Gen
Summary table
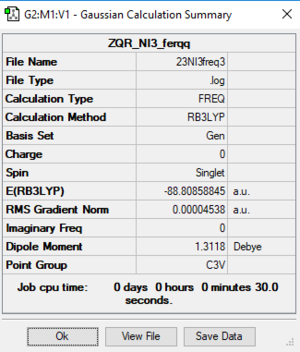
Item table
Item Value Threshold Converged? Maximum Force 0.000096 0.000450 YES RMS Force 0.000075 0.000300 YES Maximum Displacement 0.000860 0.001800 YES RMS Displacement 0.000613 0.001200 YES
frequency log file
Low frequency table
Low frequencies --- -12.7408 -12.7347 -6.4001 -0.0039 0.0188 0.0622 Low frequencies --- 101.0654 101.0662 147.4490
J mol file
NH Molecule |
Correct implementation of the pseudopotential and good structure information, however, you are missing the bond length for the molecule. Smf115 (talk) 14:51, 2 June 2019 (BST)
Mini Projects: Ionic liquids
[N(CH3)4]+ Molecule
Basic Information
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Summary table
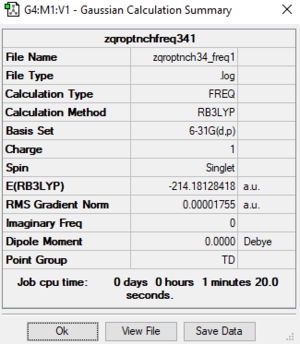
Item table
Item Value Threshold Converged? Maximum Force 0.000030 0.000450 YES RMS Force 0.000018 0.000300 YES Maximum Displacement 0.000201 0.001800 YES RMS Displacement 0.000116 0.001200 YES
Frequency log file
Low frequency table
Low frequencies --- -0.0013 -0.0011 -0.0008 21.4310 21.4310 21.4310 Low frequencies --- 188.2136 292.4076 292.4076
J mol file
BH Molecule |
[P(CH3)4]+ Molecule
Basic Information
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Summary table
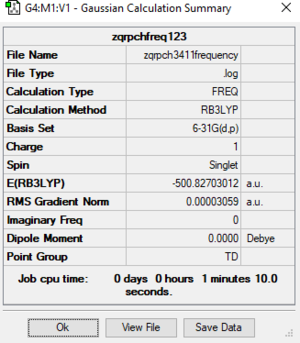
Item table
Item Value Threshold Converged? Maximum Force 0.000046 0.000450 YES RMS Force 0.000031 0.000300 YES Maximum Displacement 0.000507 0.001800 YES RMS Displacement 0.000448 0.001200 YES
Frequency log file
Low frequency table
Low frequencies --- -0.0022 0.0013 0.0025 26.7152 26.7152 26.7152 Low frequencies --- 161.3609 195.7728 195.7728
J mol file
NH Jmol |
Charge distribution
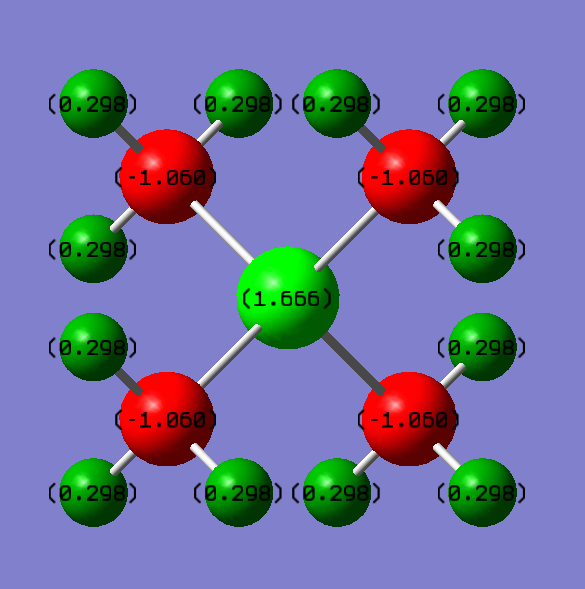

Charge on [P(CH3)4]+: P=+1.666, C=-1.050, H=+0.298.
Charge on [N(CH3)4]+: N=-0.295, C=-0.483, H=+0.269.
For [P(CH3)4]+, phosphorous is more electropositive than carbon hence most of the positive charges are concentrated on phosphorous.Therefore, the negative charges are concentrated on carbon atoms.
For [N(CH3)4]+, nitrogen is more electronegative than carbon. Therefore, the negative charge density is concentrated around nitrogen. Since carbon is also electron negative than hydrogen, the positive charge is concentrated on hydrogen atoms.
Correct NBO charges calculated and good use of a uniform colour range across both ILs, however, it should have been larger to properly show the differences between the charges. Your analysis of the charge distributions is minimal and you haven't considered the question about the +1 formal charge on the N in the traditional picture. Smf115 (talk) 20:49, 4 June 2019 (BST)
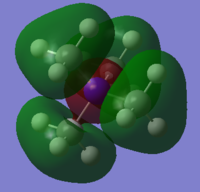
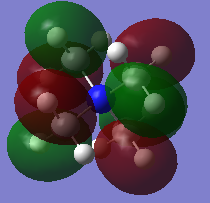
MO diagrams
Good construction of the FOs and the corresponding LCAOs, however, the last LCAO could have been drawn consistently with the other two. To improve, you could have made some attempt to consider the character of each of the MOs chosen to illustrate the range chosen. Smf115 (talk) 20:53, 4 June 2019 (BST) Overall, an ok report which could be improved by more analysis in the project section. Smf115 (talk) 20:53, 4 June 2019 (BST)| Real MO | LCAO |
 |
 |
 |
 |
 |
 |
