Yw14115
EXERCISE 1: H + H2 system
What value does the total gradient of the potential energy surface have at a minimum and at a transition structure? Briefly explain how minima and transition structures can be distinguished using the curvature of the potential energy surface.
The total gradient of the potential energy surface of the minimum are zero i.e ∂V(r)/∂r=0 ( r can be either r1 or r2). Correct Je714 (talk) 17:00, 31 May 2017 (BST)
But on the other hand, at a transition structure, the second derivative shows a positive value for different coordinate ( for both r1 and r2). In the case of using reaction coordinate, the transition structure second derivative shows negative value because it is the minima of the potential well.
Confusing explanation. You first say that at the TS the 2nd derivative is >0 for both r1 and r2 (which is wrong). I don't understand what you mean with the last sentence. Je714 (talk) 17:00, 31 May 2017 (BST)
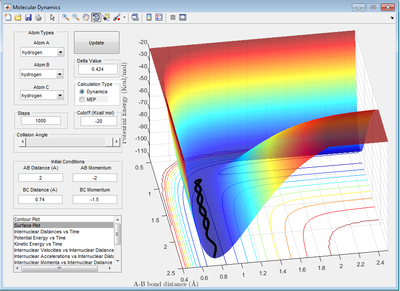
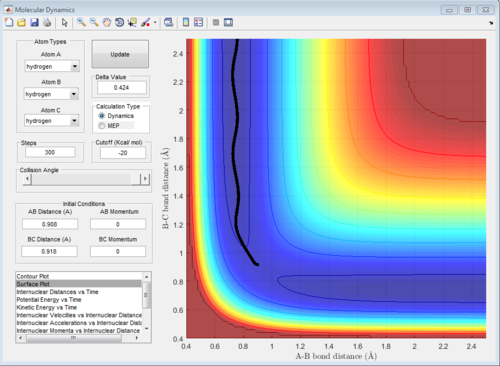
Report your best estimate of the transition state position (rts) and explain your reasoning illustrating it with a “Internuclear Distances vs Time” screenshot for a relevant trajectory.
The best estimation for the position of transition state r1 = r2 = 0.908 Da Da is Dalton units, g/mol? Do you measure the distance in such units? Je714 (talk) 17:00, 31 May 2017 (BST) . This is done by using the ' Internuclear Distance vs Time'' Graph. At the transition state, there is no oscillation for the trajectory which is shown on the graph. This is because at the transition state the first derivative of ∂u/∂r is zero which is equal to the force. As the net force is zero, there is no movement for the atoms at the transition state.
From the graph, it can be seen there is little oscillation of particles i.e the distance between A,B and B,C stays relatively unchanged.

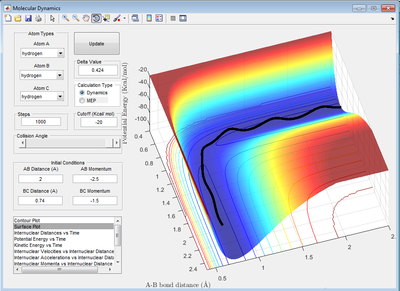
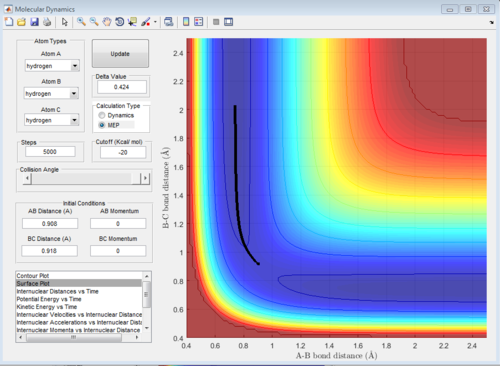
Comment on how the mep and the trajectory you just calculated differ.
For the mep, the reaction trajectory is a smooth line whereas oscillation occurs for the dynamics one. This is because the velocity of the atoms are set to zero at each time step which means there is no kinetic energy only potential energy. There is no conversion between potential energy and kinetic energy. Therefore, oscillation is not observed for the molecules. Good! Je714 (talk) 17:00, 31 May 2017 (BST)
Complete the table by adding a column reporting if the trajectory is reactive or unreactive. For each set of initial conditions, provide a screenshot of the trajectory and a small description for what happens along the trajectory.
State what are the main assumptions of Transition State Theory. Given the results you have obtained, how will Transition State Theory predictions for reaction rate values compare with experimental values?
The transition state theory is used to predict the rate of chemical reaction. The transition State theory assume the exist of activated complexes and they are in quasi equilibrium with the reactants even the classical equilibrium is not reached I see this quasi-equilibrium drawback over and over. It's not really applicable here! That is a statistical treatment of TST. In your simulations, a triatomic collision in isolation is considered -- not an ensemble of particles Je714 (talk) 17:00, 31 May 2017 (BST) . The transition state theory predicts when the reactants reach the transition state and forms the activated complex, it can either revert back to the reactants or form the product. In another word, the trajectory of PES is only allowed to cross the transition state once. Transition state theory is different form collision theory in which every successful collision lead to a reaction whereas in this case even the starting reactants have enough energy, the products still might not be formed.
In the experiment, after forming the product, it can still revert back to reactants which is the case of barrier recrossing which is different from the transition state theory. This suggest the rate of reaction in the experiment is lower than the theory predict because many high energy collisions don't lead to successful collision. Good. Recrossing is the biggest point to be made -- it's what you observe in your simulations Je714 (talk) 17:00, 31 May 2017 (BST)
EXERCISE 2: F - H - H system
PES Inspection
Classify the F + H2 and H + HF reactions according to their energetics (endothermic or exothermic). How does this relate to the bond strength of the chemical species involved? Locate the approximate position of the transition state.
For F + H2
It is an exothermic reaction which is suggested by the PES. The product channel has a lower potential energy surface compare to the reactant channel. Fluorine atom has a very high electronegativity.Therefore, H-F can form much stronger bond compare to H-H thus more energy is released for the reaction. The transition position is r1(BC)= 0.745 Da, r2(AB)= 1.811 Da. Compare to H-H-H System in which the transition state occurs between the reactant channel and product channel, the transition state for F+ H-H system occurs rather early in the reactant channel which is called early barrier. This is because the transition state region resemble the reactant structure: The F atom is still far away from the H-H and H-H bond is hardly stretched. High electronegativity of F atom makes it want to abstract the H atom.

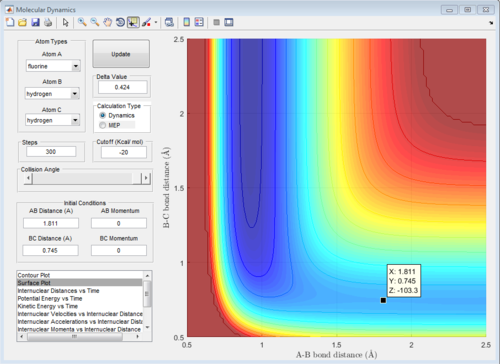
Activation energy for this reaction = 0.2 kcal/mol. it is calculated by using the energy of the transition state and the energy of the initial state.
For H + H-F
This reaction is the reversible reaction of the previous one which is endothermic. The product channel has a higher potential energy surface compare to the reactant channel. The is because H-F bond is very strong. The energy generated through H-H bond formation can not compensate the energy required to break the H-F bond thus the reaction is endothermic. The transition state position is r1(BC)= 1.811 Da r2(AB)= 0.745 Da. The transition state region resemble the structure of the product, In this case, the H atoms need to be close to each other and F atom is very far away from the H atom. The late barrier is observed for this endothermic reaction. Activation energy = 30.2 kcal/mol.

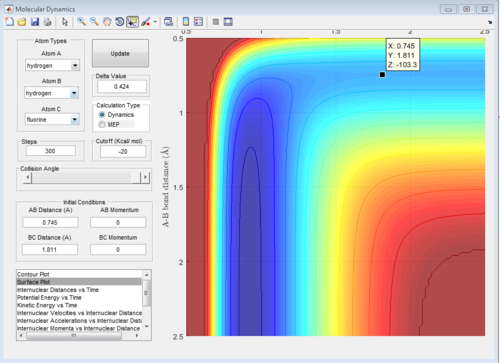
Reaction dynamic
In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?
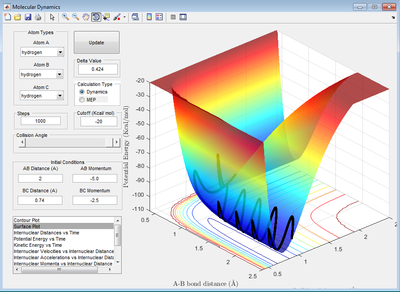
The following condition has been identified rHH = 0.75 A, rFH = 2 A and pPH = -0.5. When pHH has value between -1 and 0.5. The reactions are successful. When pHH is between -3 and -1 or 0.5 and 3, the reactions are not successful.
The input of momentum determines the total energy of the system, the total energy of the system is conserved throughout the reaction. From the graph below it can be seen the trajectory in the reactant channel has very little oscillation i.e the kinetics energy is low, the potential energy is high. After the reaction has occurred, the oscillation of the trajectory increase in a huge extant. This is because a lot of potential energy has transferred into kinetic energy because the total energy of the system must be conserved. This experimental observation is evident by the following graph. Missing discussion about how you would set up an experiment to confirm this... Je714 (talk) 17:00, 31 May 2017 (BST)

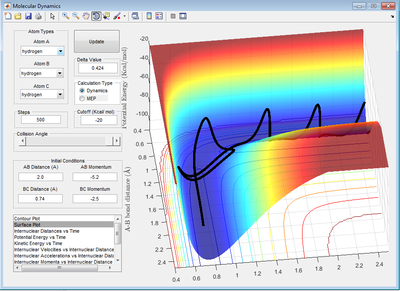
Discuss how the distribution of energy between different modes (translation and vibration) affect the efficiency of the reaction, and how this is influenced by the position of the transition state
Polanyi's empirical rules state translational energy is most efficient for a exothermic reaction which has a early barrier than vibrational energy. In this case, energy is needed in the reactant channel to cross the barrier. when the relative translational energy is higher than vibrational energy, then there is enough kinetic energy available in the reactant channel which means it can cross the barrier in the reactant channel and the reaction is possible to occur. After crossing the transition state, the trajectory can climb up the potential well and initiate vibrations of product molecule. Therefore, a vibrating trajectory is usually observed in the product channel. If the barrier is in the product channel, the trajectory would just simple bounce off from the potential well, the reaction wouldn't occur. On the other hand, vibrational energy is most efficient for an endothermic reaction which has late barrier. When the relative vibrational energy is higher than the translational energy, the kinetic energy is available along the product channel to go over the late barrier.
In both cases, the total energy should be greater than the energy of the barrier to make the reaction favourable.
You need to show examples of your trajectories for each case! Not complete. Je714 (talk) 17:00, 31 May 2017 (BST)