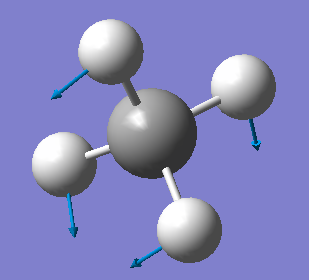
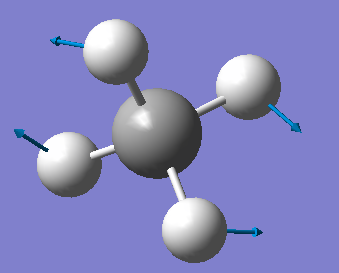
Yt6718
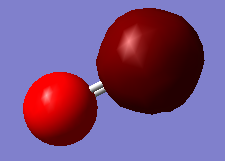
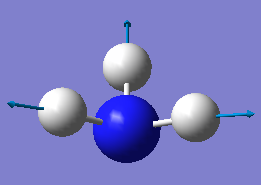
NH3 optimisation
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -56.55776871 a.u.
RMS Gradient Norm = 0.00004473 a.u.
Point Group = C3V
optimised N-H bond distance=1.01806Å
optimised H-N-H bond angle=105.747°
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.000080 | 0.000450 | YES |
| RMS Force | 0.000053 | 0.000300 | YES |
| Maximum Displacement | 0.000190 | 0.001800 | YES |
| RMS Displacement | 0.000124 | 0.001200 | YES |
Predicted change in Energy=-2.197407D-08
test molecule |
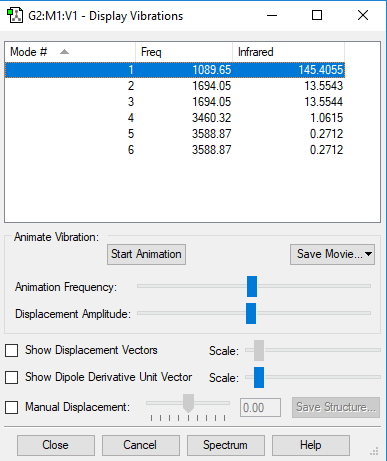
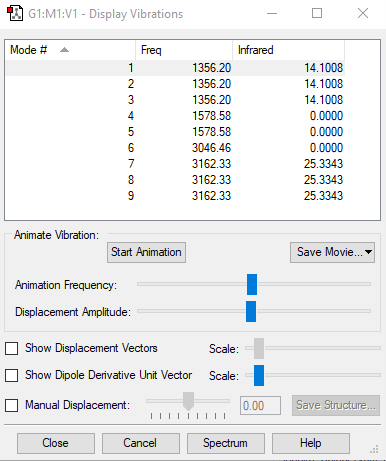
| wavenumber cm-1 | 1089.6540 | 1694.0484 | 1694.0484 | 3460.3171 | 3588.8744 | 3588.8744 |
| symmetry | A1 | E | E | A1 | E | E |
| intensity
(arbitrary units) |
145.4055 | 13.5543 | 13.5544 | 1.0615 | 0.2712 | 0.2712 |
| image | 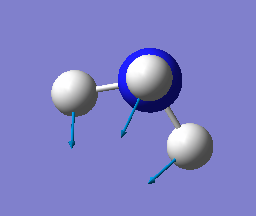
|
|
|
|
|
|
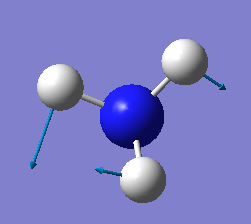
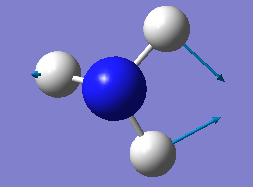
how many modes do you expect from the 3N-6 rule? 6
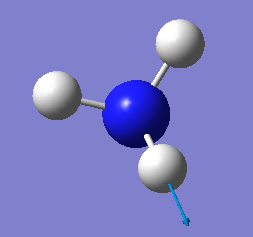
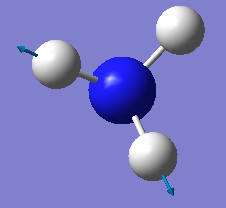
which modes are degenerate (ie have the same energy)? two modes of frequency at 1694
which modes are "bending" vibrations and which are "bond stretch" vibrations? first three modes are bending vibrations and last three modes are bond stretch vibrations
which mode is highly symmetric? No.4 mode
one mode is known as the "umbrella" mode, which one is this? mode 1
how many bands would you expect to see in an experimental spectrum of gaseous ammonia? 4
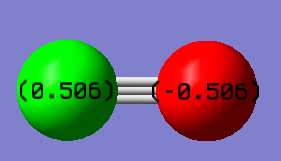
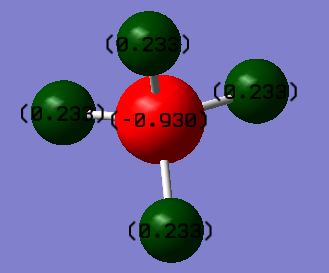
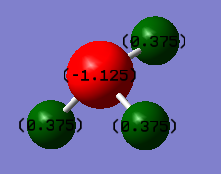 charge positive for H and charge negative for N atom. Because N atom is more electronegativity than H atom and it will attract electron pairs closer to it.
charge positive for H and charge negative for N atom. Because N atom is more electronegativity than H atom and it will attract electron pairs closer to it.
N2 optimisation
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -109.52359111 a.u.
RMS Gradient Norm = 0.02473091 a.u.
Point Group = D∞h
optimised N-N bond distance=1.10550Å
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.042835 | 0.000450 | NO |
| RMS Force | 0.042835 | 0.000300 | NO |
| Maximum Displacement | 0.011569 | 0.001800 | NO |
| RMS Displacement | 0.016360 | 0.001200 | NO |
Predicted change in Energy=-4.958069D-04
test molecule |
| wavenumber cm-1 | 2457.3283 |
| symmetry | SGG |
| intensity
(arbitrary units) |
0.0000 |
| image | 
|
H2 optimisation
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -1.17853936 a.u.
RMS Gradient Norm = 0.00000017 a.u.
Point Group = D∞h
optimised H-H bond distance=0.74279Å
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.168347 | 0.000450 | NO |
| RMS Force | 0.168347 | 0.000300 | NO |
| Maximum Displacement | 0.119698 | 0.001800 | NO |
| RMS Displacement | 0.169278 | 0.001200 | NO |
Predicted change in Energy=-2.130559D-02
test molecule |
| wavenumber cm-1 | 4465.6824 |
| symmetry | SGG |
| intensity
(arbitrary units) |
0.0000 |
| image | 
|
Sturcture ABASUR[here] has H-H bond length 0.99435Å and computer simulation gets 0.74279Å. This difference may because of the angle strain fron the compound and different chemical environments. Also, in the compound H forms two bounds while pure H2 gas only forms one bond.
E(NH3)= -56.55776871 a.u. 2*E(NH3)= -113.1155374 a.u. E(N2)= -109.52359111 a.u. E(H2)= -1.17853936 a.u. 3*E(H2)= -3.53561808 a.u. ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -148.2 kJ/mol
The energy for converting hydrogen and nitrogen gas into ammonia gas is -148.2 kJ/mol. Ammonia productis are more stable because it is an exothermic reaction the product is lower in energy states and thermodinamicly more stable.
CO optimisation
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -113.30945314 a.u.
RMS Gradient Norm = 0.00001828 a.u.
Point Group = C∞V
optimised C=O bond distance=1.13793Å
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.191096 | 0.000450 | NO |
| RMS Force | 0.191096 | 0.000300 | NO |
| Maximum Displacement | 0.113036 | 0.001800 | NO |
| RMS Displacement | 0.159857 | 0.001200 | NO |
Predicted change in Energy=-2.270461D-02
test molecule |
| wavenumber cm-1 | 2209.1389 |
| symmetry | SG |
| intensity
(arbitrary units) |
67.9587 |
| image | 
|
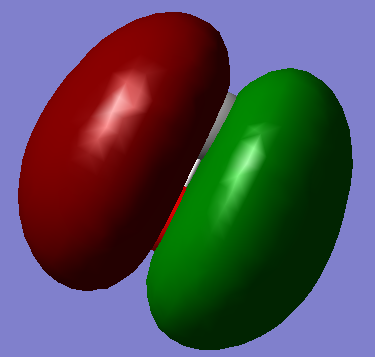
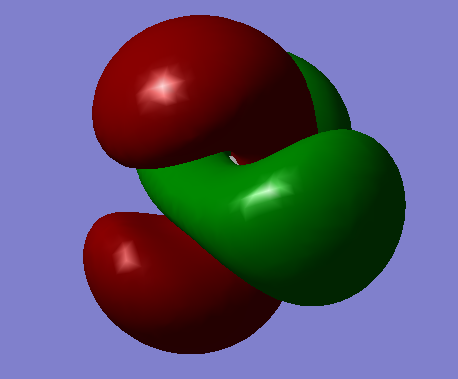
This HOMO gerada sigma bonding forms by one electron from C's 2P orbital and one electron from O's 2P.
This gerada pi bonding forms by one electron from C's 2P orbital and three electrons from O's 2P.
This ungerada sigma anti-bonding forms by one electron from C's 2S orbital and one electron from O's 2S.
This gerada sigma bonding forms by one electron from C's 2S orbital and one electron from O's 2S.
This ungerada sigma anti-bonding forms by one electron from C's 1S orbital and one electron from O's 1S.
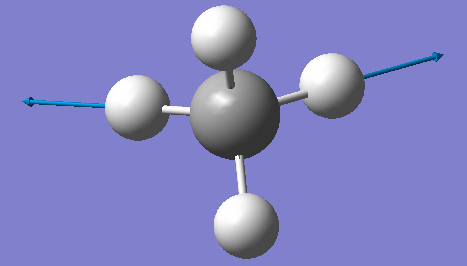
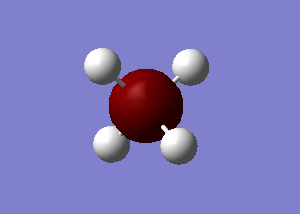
CH4 optimisation
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
E(RB3LYP) = -40.52401404 a.u.
RMS Gradient Norm = 0.00003263 a.u.
Point Group = TD
optimised C-H bond distance=1.09197Å
optimised H-C-H bond angle=109.471
| Item | Value | Threshold | Converged? |
|---|---|---|---|
| Maximum Force | 0.015565 | 0.000450 | NO |
| RMS Force | 0.008320 | 0.000300 | NO |
| Maximum Displacement | 0.041520 | 0.001800 | NO |
| RMS Displacement | 0.022194 | 0.001200 | NO |
Predicted change in Energy=-1.301482D-03
test molecule |
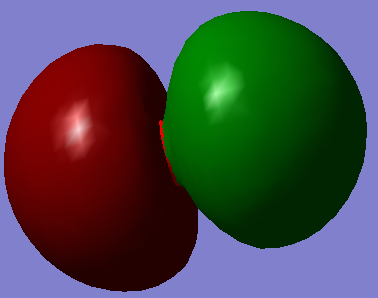
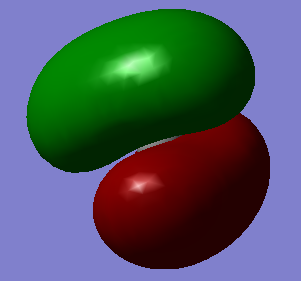
This LOMO unoccupied gerada pi bonding bond.
This HOMO gerada sigma bonding forms by two electron from H and four electrons from C's 2P orbital.
This ungerada sigma anti-bonding forms by one electron from C's 1S orbital and one electron from H.
This gerada sigma bonding forms by one electron from C's 1S orbital and one electron from one of the H's 1S.
This un occupied ungerada pi anti-bonding..
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
You could have used subheadings more efficiently and you labelled all jmols as 'test molecule' which is not informative at all.
NH3 0/1
Have you completed the calculation and given a link to the file?
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Have you included summary and item tables in your wiki?
YES
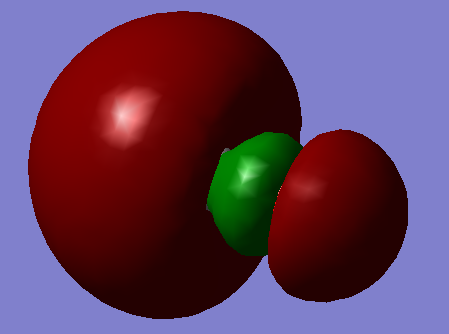
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - You correctly identified two sets of degenerate modes which explains a spectrum with 4 bands. All stretching vibrations are oo low in intensity to be observed, so only 2 bands are expected in an experimental spectrum.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES - however your overall result is wrong by 3 kJ/mol which may originate from rounding during the calculation.
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 1.5/5
Have you completed the calculation and included all relevant information?
NO -You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Have you added information about MOs and charges on atoms?
You have not commented on the calculated charges or vibrations at all. You stated the MOs to originate from AOs electrons. However, the AOs only originate from mixing the AOs and the occupancy is a result of Hund's rule. Youcorrectly identified the contributing AOs - well done! You missed to comment on the occupancy of the MOs and the relative order in terms of energy. You could have more specific stating contributing AOs by labelling p orbitals with their orientation. The last displayed MO is a non-bonding MO as it only is originating from the 2s orbital of carbon.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES - But you missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Do some deeper analysis on your results so far