Yr2Mod:j cos inorganic 2019
Day 1
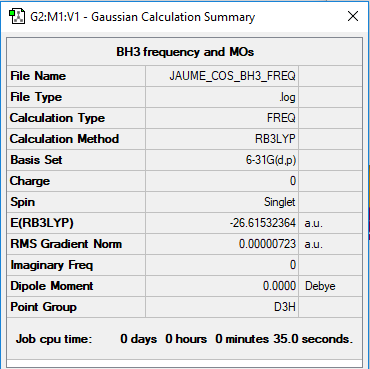
BH3 Borane link
B3LYP/6-31G(d,p)
Low frequencies and convergence:
Low frequencies --- -15.9849 -15.9818 -12.7207 0.0006 0.0143 0.3137
Low frequencies --- 1162.9365 1213.1134 1213.1136
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000057 0.001800 YES
RMS Displacement 0.000028 0.001200 YES
Jmol:
BH3 |
Vibration analysis:
| Vibrational mode number | IR Frequency (cm^-1) | Type |
|---|---|---|
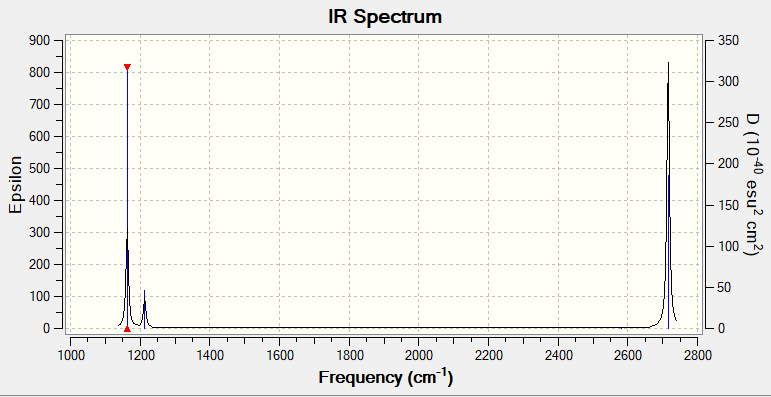
| 1 | 92.5721 | Symmetric out-of-plane bend |
| 2 | 14.0532 | Symmetric wag |
| 3 | 14.0527 | Asymmetric wag |
| 4 | 0.0000 | Symmetric stretch (inactive) |
| 5 | 126.3274 | Asymmetric stretch |
| 6 | 126.3214 | Asymmetric stretch |
Answers to questions about vibrations
While the molecule has 6 vibrational modes, however only three peaks are visible in the IR spectrum. This is due to there being one non-degenerate IR-active mode (1), two pairs of denegerate IR-active modes (2&3, 5&6) and one IR-inactive mode (4.) (it is inactive due to having no change in dipole moment).
Correct reasons are given for the number of visible peaks but they could have been explained a bit better. There are some mistakes in the information reported (e.g. mix up between frequencies and IR intensity) and you haven't thought about the symmetry of the modes. Smf115 (talk) 08:09, 13 May 2019 (BST)
MO Diagram for BH3
Lecture_4_Tut_MO_diagram_BH3,http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf, P.Hunt, accessed 03/05/2019
Answers to questions about MOs
As can be seen in this diagram, the qualitative MO diagram obtained by LCAO, is a close qualitative fit of the actual orbitals calculated computationally. While LCAO cannot give reliable numerical solutions, it can closely and accurately predict the electron distribution in real MOs.
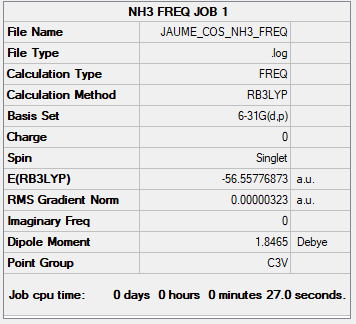
NH3 Ammonia link
B3LYP/6-31G(d,p)
Low frequencies and convergence:
Low frequencies --- -0.0131 -0.0028 -0.0019 7.0747 8.1044 8.1047
Low frequencies --- 1089.3849 1693.9369 1693.9369
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000013 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Jmol:
NH3 |
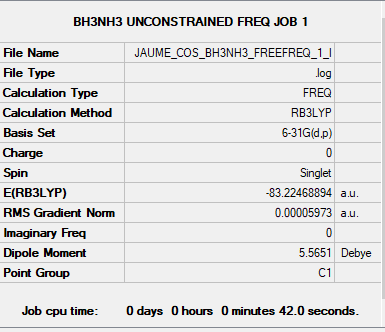
BH3NH3 link
B3LYP/6-31G(d,p)
Low frequencies and convergence:
Low frequencies --- -0.0006 0.0002 0.0010 16.8256 17.3871 37.9705
Low frequencies --- 265.9699 632.2062 639.3209
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000013 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Jmol:
BH3NH3 |
Energetic Calculations
ΔE = E[BH3NH3] - (E[BH3] + E[NH3])
-83.22468894 a.u. - (-26.61532364 a.u. + -56.55776873 a.u.) = -0.05159657 a.u
ans*2625.5 kJ/mol = -135 kJ/mol
Normal B-N bond energy is 389 kJ/mol, our B-N dative bond is weak.
Correct calculation, energy conversion and consideration of the accuracy of the final result. Good comparison but your literature value lacks a literature reference!Smf115 (talk) 08:12, 13 May 2019 (BST)
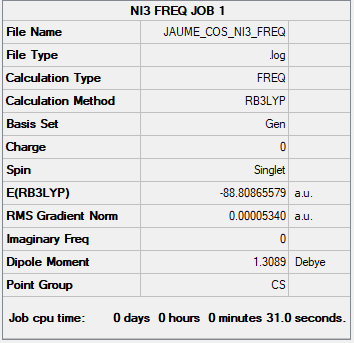
NI3 link
B3LYP/6-31G(d,p) ; pseudo-potential LANL2DZ
Low frequencies and convergence:
Low frequencies --- -7.1402 -4.8083 -3.3178 -0.0004 -0.0003 -0.0001
Low frequencies --- 101.0869 101.1948 148.1919
Item Value Threshold Converged?
Maximum Force 0.000133 0.000450 YES
RMS Force 0.000053 0.000300 YES
Maximum Displacement 0.001104 0.001800 YES
RMS Displacement 0.000471 0.001200 YES
Jmol:
NI3 |
Optimised N-I distance
The optimised N-I distance was found to be 2.18293 Å.
Day 2 and 3 Mini-Project: Lewis Acids
Energy Calculations
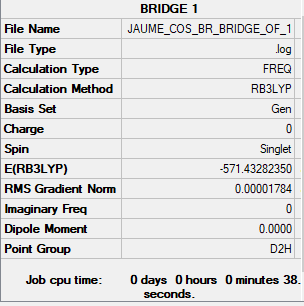
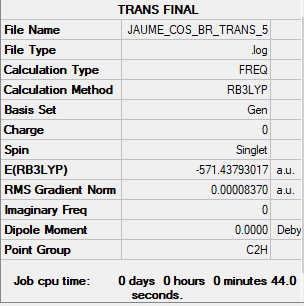
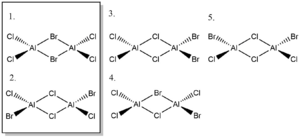
Comparing the energies of the two isomers of Al2Cl4Br2:
E[Al2Cl4Br2](Br bridge)= -571.43282350 a.u. E[Al2Cl4Br2](Br trans)= -571.43793017 a.u. The difference in energy between the two isomers is: 13 kJ/mol
The isomer with bridging Cl and trans terminal Br is lower in energy. Since Br has n quantum number n=4, it is large, and having the Br be terminal trans to each other minimises repulsion while having bridging Br maximises it, leading to higher energy.
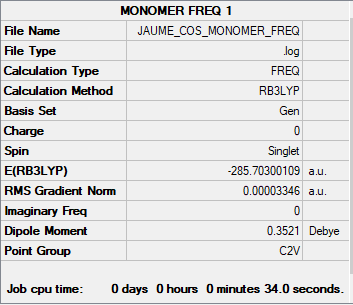
Obtaining the dissociation energy of the Al2Cl4Br2</sub dimer:
ΔE = E[Al2Cl4Br2] - 2(E[AlCl2Br]) ΔE = -571.43793017 a.u. - 2(-285.70300109 a.u.)
ΔE = -0.003192799 a.u. ΔE = -0.003192799 a.u. * 2625.5 kJ/mol = -84 kJ/mol
Dissociation energy of this Dimer is 84 kJ/mol. Its dimer form is favoured with respect to its monomer form. Cl and Br are electronegative elements, thus, forming a dimer in which the most electronegative of the two, Cl (3.16 cf. 2.6) is bridging is favoured.
Good assignment of the symmetries to all 5 isomers. The calculations are in general good, however, you've calculated the association energy not dissociation and your energies are incorrect due to the incorrect pseudopotential input. Smf115 (talk) 08:16, 13 May 2019 (BST)
Al2Cl4Br2 with bridging Br link
GEN; N B3LYP/6-31G(d,p) , pseudo-potential Br Cl LANL2DZ
Low frequencies and convergence:
Low frequencies --- -1.1123 0.0001 0.0007 0.0007 1.0417 1.8073
Low frequencies --- 16.1837 62.5427 84.8372
Item Value Threshold Converged?
Maximum Force 0.000045 0.000450 YES
RMS Force 0.000020 0.000300 YES
Maximum Displacement 0.001233 0.001800 YES
RMS Displacement 0.000523 0.001200 YES
Jmol:
Al2Cl4Br2 |
Al2Cl4Br2 with trans terminal Br and bridging Cl link
GEN; N B3LYP/6-31G(d,p) , pseudo-potential Br Cl LANL2DZ
Low frequencies and convergence:
Low frequencies --- -4.3978 -2.4768 -1.5835 -0.0010 0.0004 0.0007 Low frequencies --- 18.6602 47.6012 71.2010 Item Value Threshold Converged? Maximum Force 0.000244 0.000450 YES RMS Force 0.000084 0.000300 YES Maximum Displacement 0.002000 0.001800 NO RMS Displacement 0.000849 0.001200 YES
This output file shows that the Maximum Displacement has not converged; this is due to the fact that the basis set that we're using is not accurate enough for calculations of this molecule.
As Br is quite diffuse, the gradient of the maximum displacement is not close enough to zero.
In any case, this is to be expected considering the approximations that are being used, however the Maximum Displacement gradient is small enough that we can use these results.
Nice to see this awareness and addressing it in your report! Great to see the pseudopotential correctly implemented for Br, however, you weren't meant to include it on the Cl which is where the likely convergence issue has probably arisen. Smf115 (talk) 08:16, 13 May 2019 (BST)
Jmol:
Al2Cl4Br2 |
AlCl2Br Monomer link
GEN; N B3LYP/6-31G(d,p) , pseudo-potential Br Cl LANL2DZ
Low frequencies and convergence:
Low frequencies --- -0.0009 -0.0009 0.0007 1.3337 2.3401 4.2420
Low frequencies --- 119.7829 132.7164 182.6113
Item Value Threshold Converged?
Maximum Force 0.000084 0.000450 YES
RMS Force 0.000033 0.000300 YES
Maximum Displacement 0.001205 0.001800 YES
RMS Displacement 0.000690 0.001200 YES
Jmol:
AlCl2Br |
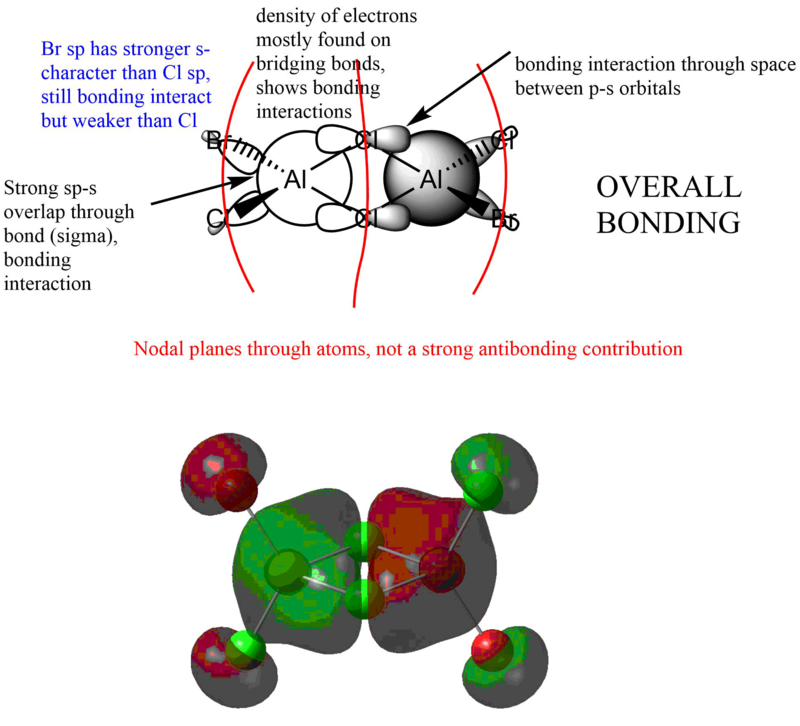
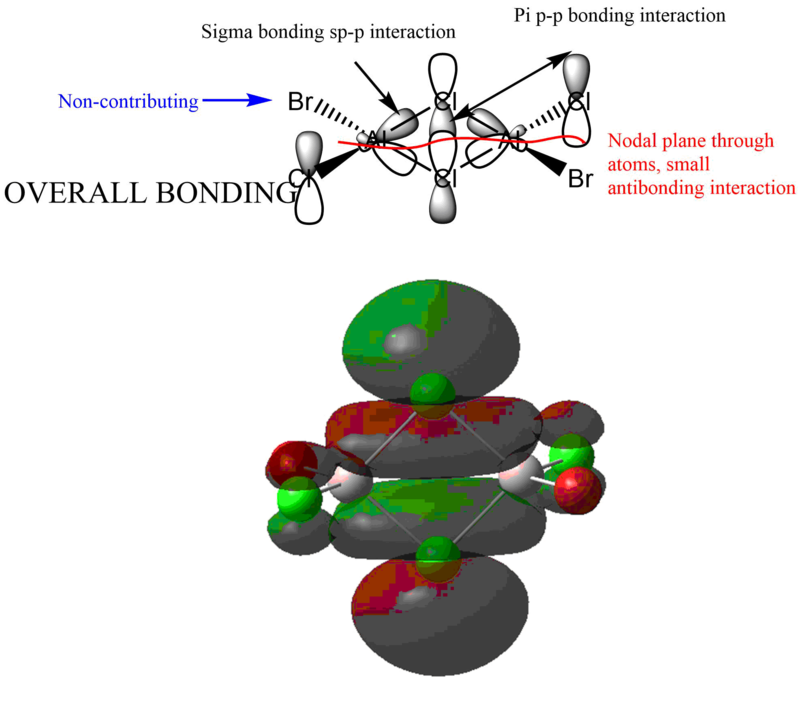
MO analysis of lower energy isomer (trans terminal Br)
Good range of MOs selected and you've evaluated the overall bonding/anti-bonding character well. Your LCAO diagrams are ok but there are a few mistakes, such as whether the Al contributes to the MO at all. Really good attempt at labelling some of the interactions and nodal planes, to improve though you've missed out quite a few interactions and the presentation of the annotations could be clearer .Smf115 (talk) 08:21, 13 May 2019 (BST)
Overall, good report and great effort across both sections, particularly all the structures and optimisation information is good. Smf115 (talk) 08:21, 13 May 2019 (BST)