Yg5515
Introduction
Potential energy surface is important in discovering the relationship between molecular energies and the geometry. It formulated on the basis of Born-Oppenheimer approximation which states that nuclei are fixed in positions and not exchanging energies with electrons. Extracting and calculating information from the PES, one could investigate more about the reaction dynamics. Th first part of this lab focus on the H2+H reaction, in which one hydrogen atom approach and attack the hydrogen molecule, forming a new hydrogen molecule and kicking out one hydrogen away. Transition state is located and the limitation of Transition state theory is investigated via exploring different reaction trajectories. The second part is focused on the H-F-H system. Transition states, activation energies and reaction dynamics are investigated.

Exercise 1 H+H2
Question 1
The lowest energy pathway which links the two minimas, i.e. Ha and Hbc was the reaction coordinates. As the reaction proceeds follow the energy minima pathway, it will go through a energy maxima which is the transition state.
Both energy minimas and transition state will have total gradients zero, as they are stationary points. In order to differentiate a transition state from energy minimas, second derivatives are needed.
From figure 3 , it can be seen that transition state is located at a saddle point, which is a maxima along the reaction coordinate, but is a minima in all the other directions.
 |
 |
From the view along the pathway, it can be seen that product and reactant are at the minima and the transition state is at maximum.
Question 2
locating transition state
By setting r2-=r2 and p1=p0, animation shows that the Ha and Hc are symmetrically stretching with respect to Hb. It was found that r ts=0.908. This is when the molecules start to freeze and the internuclear distance v.s. time graph shows two horizontal lines. Hac distance was stablized at 1.81 and Hbc or H ac distance was at 0.908, exactly half of of distance of Hac.
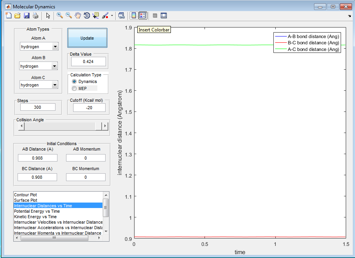 |
 |
To confirm, the Internuclear distance- time graph with original setup is plotted. Transition state exists at the cross point of two lines, this is when the internuclear distances between AB and BC the same.The point is at r=0.906.
Question 3
To investigate the minimum energy pathway, the r1 was set to be 0.918, plus 0.01 to the rst and r2 was set to equal to r2. Figure 6 shows that the reaction pathway, i.e. the black line is smooth and only the path which corresponds to the formation of the new H2 bond can be seen.

Firstly, Atom C moving towards right, forming the transition state. Then molecule BC formed and Atom C moving away. This explains Figure 9, which AC internuclear distance is increasing and it has the maximum internuclear distance among the three distances, as the bond is formed between Atom BC, the distance is decreasing.
 |
 |
It can be seen that the reaction pathway for molecular dynamics has more wavelike property which indicates that it has taken into account of the motion of the molecules, whereas, MEP showed a smooth line, assuming that molecule and atoms do not have self motions.
Internuclear distance vs time and Internuclear momenta vs time are compared for calculation types.
Question 4
This question investigate the conditions for reactive trajectory. It is concluded that for a reactive trajectory, the conditions are: r1=0.74, r2=2.0, -1.5<p1<-1.8, p2=-2.5. Trajectories which have the same r values but higher momenta are all expected to be reactive. However, sometimes, transition state recross may happen when P2 is large. Consequently, this may lead to overall an unreactive trajectory. The table below compares five set of combinations of P1 and P2 values. The last two sets involves TS recrossing.

Transition State theory
Transition state theory states that there is an equilibrium between the activated complex and the reactants and the rate at which it passes transition state determines the rate at which the transition state forms the products. The theory is based on classical mechanics which implies that only collisions which have sufficient energy to overcome the activation energy will result in the formation of products. However, from the point view of quantum mechanics, particles are able to overcome small energy barriers by tunneling. This means even though the molecules do not have enough energies, collisions may still results in product formation. Experimental results deviated from the theoretical predictions when the reaction has small energy barrier. At high temperature, more particles are populated at higher energy level,therefore and the vibrational modes are very complex which makes the reactivity of the transition state differs from the theoreticals. The TST also assumes negligible recrossing, which is in contradictory to the experimental results. Overall, the experimental rate will be lower than those of theoreticals.
Exercise 2 F-H-F system
H-F-H
Two reactions, H2-F and H-F2 are investigated. It should be noted that the two reactions are the reverse of each other, therefore, one of them is exothermic and the other is endothermic. In addition, both of the reactions share the same transition state, although according to Hammond postulate, forward reaction has early transition state and backward reaction has late transition state. Overall, the transition state resembles, H2+F. H-F bond is stronger than H2, therefore, for H2+F, the formation of H-F releases large amount of energy which outweighs the energy neeed to break the H2, resulting in exothermic reaction.
TS r1=1.809, r2=0.744, E=-103.3 Kcal/mol
 |
 |
 |
 |
Ea of F+H2:0.5 Kcal/mol Ea of H+HF:30.3 Kcal/mol
reaction dynamics
The conditions of H2-F reactive trajectory are investigated. rHH :0.74 momenta of H-F:-0.5, momenta of H-H was set in the range of -3 to 3.
 |
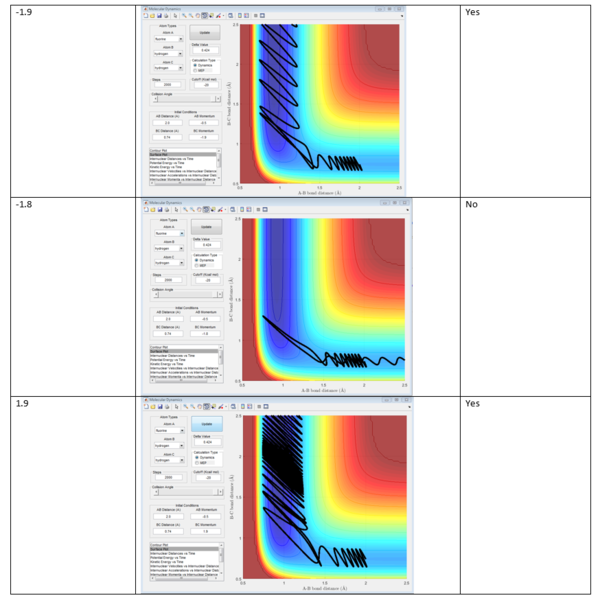 |
 |
As can be seen from the table, when the momentum of H-H was too large, bigger than 2.4 or -2.4, the reaction trajectory is unreactive,because the larger the momentum of the H-H bond, the stronger the vibrations and the greater the kinetic energy it has. Compare with the little translational momentum that H-F possesses, the H-H momentum dominates the overall momentum which lies heavily on H-H. This makes F atom fail to interact with H-H and consequently leads to unreactive trajectory. Now the initial conditions has changed, P F-H is -0.8 and P H-H is 0.1. Reactive trajectory can be seen because the momentum of P F-H has increased which makes the F atom has more translational momentum.
|

H+HF system Polanyi emperical rule state that for a reaction which has late transition state, such as an endothermic reaction, vibrational energy plays a more important role in promoting reactions than translational energy. This can be demonstrated by comparing two set of experimental data, one of which has very large virational energy by setting the momentum of H-F very high, other of which has very high translatonal momentum of the incoming H.
Comparing two figures, it can be concluded that, for a reaction which has late energy barrier, vibrational energy contributes more than translational energy for the promotion of reactive trajectory.
Conclusion
Transition state is the saddle point of the potential energy surface. According to Transition state theory, the reaction must proceeds via transition state, however, in reality,quantum behavior, such as tunneling can happen, which makes the theoretical values higher than the experimental. The theory also fail to explain transition state recrossing. By investigating H-F-H system, Polanyi emperical rule can be proved.
(A good report but I think you skipped a question "In light of the fact that energy is conserved, discuss the mechanism of release of the reaction energy. How could this be confirmed experimentally?" Lt912 (talk) 01:51, 26 May 2017 (BST))






