Yg1333614
NH3 molecule
NH3 molecule |
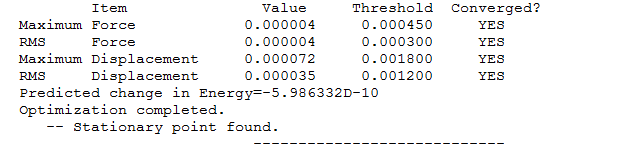
The optimisation file is liked to here
Information of NH3 molecule
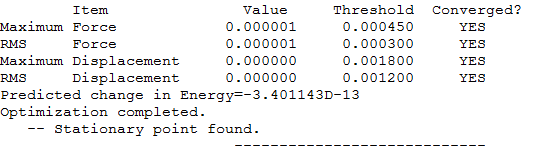
Calculation Type = FREQ
Calculation Method = RB3LYP
Basis Set = 6-31G(d,p)
Charge = 0
Spin = Singlet
E(RB3LYP) = -56.55776873 a.u.
RMS Gradient Norm = 0.00000485 a.u.
Imaginary Freq = 0
Dipole Moment = 1.8466 Debye
Point Group = C3V
Bond Angles=105.741
Bond Length=1.01798
Raw data of NH3
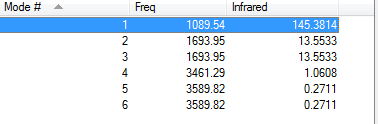
Vibration data of NH3
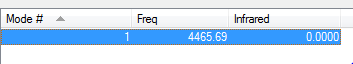
There are 6 unique vibration modes of a NH3 molecule shown as followings:
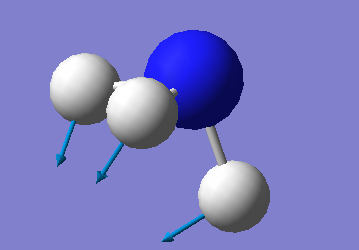
The one shown below is a model of the most common vibrational mode:
There are 6 modes from the calculation 3*4-6=6
The modes are not degenerate since they have different energy
Only the first mode and the third mode are bending modes, the rest are stretching modes.
The 5th and 6th modes are highly symmetric because they have the least vibrational energy value
The first mode is the umbrella mode
Only one band should be seen in IR spectra,because there is only one type of N-H bond in this molecule
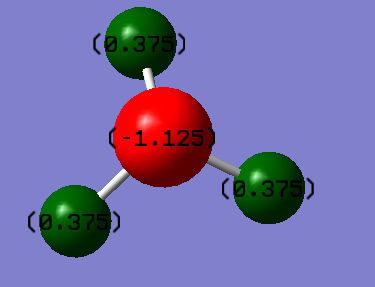
Charge distribution of NH3
due to the difference in electronegativity of different atoms in a NH3 molecule, the charges do not distribute evenly.The ammonia molecule have a charge distribution shown below:
Reaction energies of NH3
Ammonia molecule could be generated through Haber Process 3H2+N2->2NH3. The reaction energies of NH3, the reactants and the difference in energies in this reaction is shown below:
E(N2)=-109.52412868 a.u.
3*E(H2)=-3*1.17853936 a.u.=-3.535 a.u.
2*E(NH3)=-2*56.55776873 a.u.=-113.115 a.u.
delta E=2*E(NH3)-[E(N2)+3*E(H2)]=-113.115-(-109.524-3.535)=-0.056 a.u.
The negative sign indicates that this reaction process is exothermic and the final products of ammonia is more stable than the initial reactants.
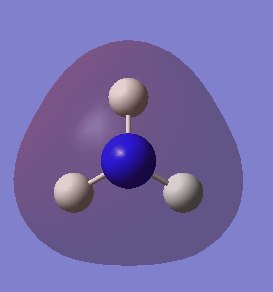
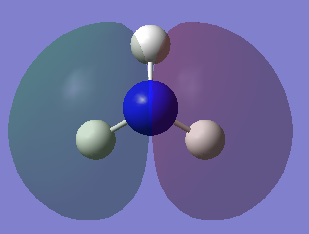
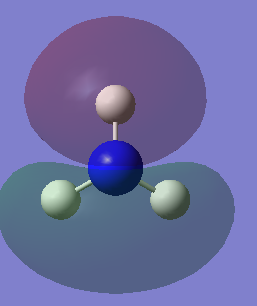
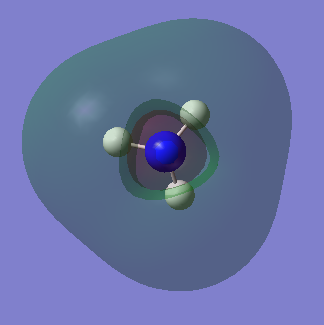
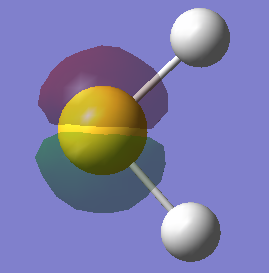
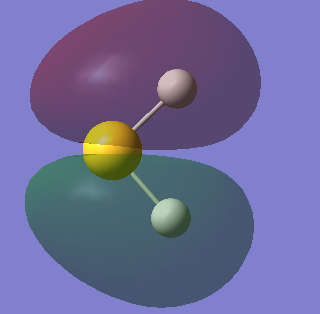
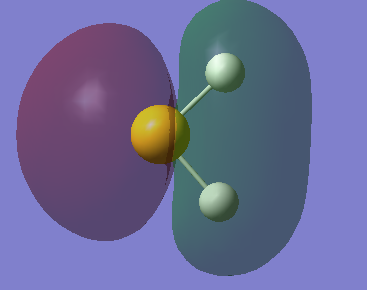
Molecular orbitals of NH3
N2 molecule
N2 molecule |
The optimisation file is liked to here
information of N2 molecule
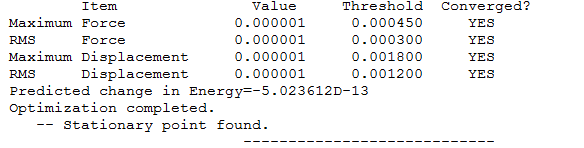
Calculation Type=FREQ
Calculation Method=RB3LYP
Basis Set=6-31G(d,p)
Charge=0
Spin=Singlet
E(RB3LYP)=-109.52412868 a.u.
RMS Gradient Norm=0.00000060 a.u.
Imaginary Freq=0
Dipole Moment=0.0000 Debye
Point Group=D*H
Bond length=1.10550
Bond angle=180
Raw data of N2 molecule
Vibration data of N2 molecule
H2 molecule
H2 molecule |
The optimisation file is liked to here
Information of H2 molecule
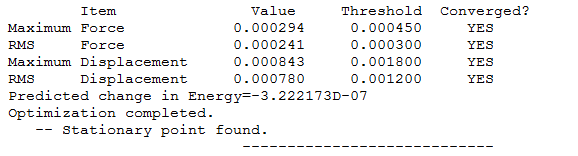
Calculation Type=FREQ
Calculation Method=RB3LYP
Basis Set=6-31G(d,p)
Charge=0
Spin=Singlet
E(RB3LYP)=-1.17853936 a.u.
RMS Gradient Norm=0.00000036 a.u.
Imaginary Freq=0
Dipole Moment=0.0000 Debye
Point Group=D*H
Bond length=0.74279
Bond angle=180
Raw data of H2 molecule
Vibration data of H2 molecule
H2S molecule
H2S molecule |
The optimisation file is liked to here
information of H2S molecule
Calculation Type=FREQ
Calculation Method=RB3LYP
Basis Set=6-31G(d,p)
Charge=0
Spin=Singlet
E(RB3LYP)=-399.39162393 a.u.
RMS Gradient Norm=0.00018900 a.u.
Imaginary Freq=0
Dipole Moment=1.3993 Debye
Point Group=C2V
Bond angle=92.65959
Bond length=1.34714
Raw data of H2S molecule
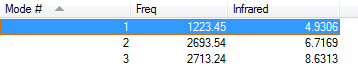
Vibration data of H2S
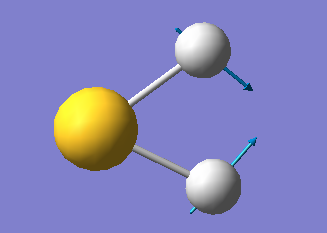
There are only three modes of vibration of H2S molecule, they are shown below:
The one mode shown below is a bending vibration of H2S molecule.
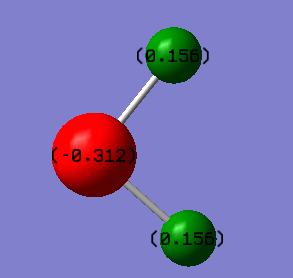
Charge distribution of H2S molecule
Due to the difference in electronegativity in different molecules in H2S, the charges are distributed unevenly, as shown below: