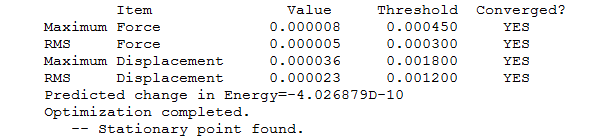Year 2 Computational Labs Eleonora
Year 2 Inorganic Computational Lab
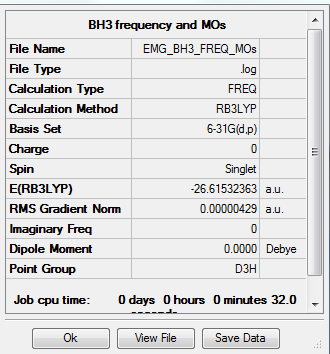
BH3
Calculation method = RB3LYP
Basis = 6-31G(d.p)
Summary table for optimised molecule
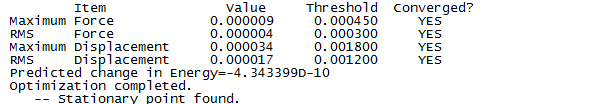
"Item" table for optimised molecule
Link to frequency log file and low frequency lines table
Low frequencies --- -2.2126 -1.0751 -0.0054 2.2359 10.2633 10.3194 Low frequencies --- 1162.9860 1213.1757 1213.1784
Jmol image from frequency file
optimised BH3 molecule |
Smf115 (talk) 22:48, 16 May 2018 (BST)Well presented wiki report and structure information throughout.
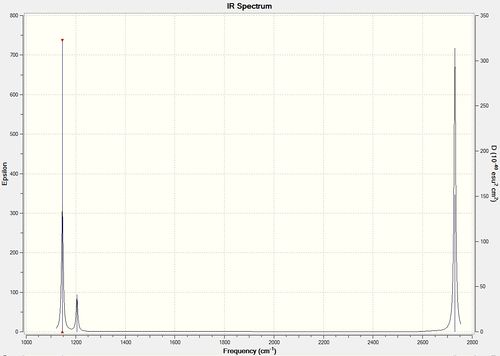
Vibrational spectrum for BH3
| wavenumber (cm-1) | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A2" | yes | out-of-plane bend |
| 1213 | 14 | E' | very slight | bend |
| 1213 | 14 | E' | very slight | bend |
| 2582 | 0 | A1' | no | symmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
| 2715 | 126 | E' | yes | asymmetric stretch |
When comparing the IR spectrum to the IR table, it can be seen that the number of peaks do not correspond. This is due to one of the IR values originating from a symmetrical stretch, therefore making it IR inactive, and due to there being two pairs of degenerate vibrational frequencies.
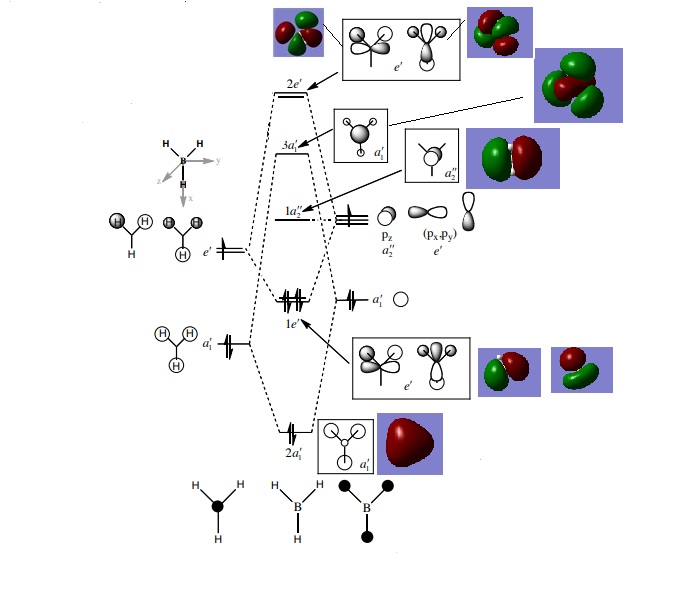
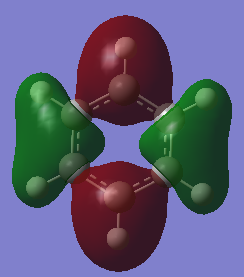
MO diagram for BH3
As can be seen from the MO diagrams, the linear combination of atomic orbitals gives a rough yet accurate vision of the molecular orbitals. The volumes of the orbitals are significantly smaller than those of the MO orbitals however the positions are correct. (Reference: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf)
Smf115 (talk) 22:47, 16 May 2018 (BST)Clear inclusion of the MOs and good mention of the subtle differences between the orbitals.
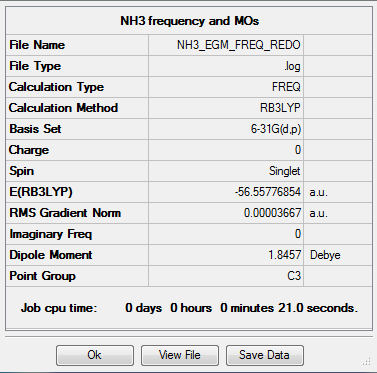
NH3
Calculation method = RB3LYP
Basis = 6-31G(d.p)
Summary table for optimised molecule
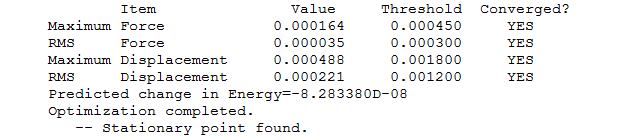
"Item" table for optimised molecule
Link to frequency log file and low frequency lines table
Low frequencies --- -30.6570 -30.6559 -0.0052 0.0121 0.0479 4.3582 Low frequencies --- 1088.6591 1694.0274 1694.0274
Jmol image from your frequency file
optimised NH3 molecule |
Ammonia-borane
Calculation method = RB3LYP
Basis = 6-31G(d.p)
Summary table for optimised molecule
"Item" table for optimised molecule
Link to frequency log file and low frequency lines table
Low frequencies --- -183.2938 -66.8079 -66.7947 -0.0053 0.0093 0.0138 Low frequencies --- 202.6720 660.5161 660.5187
Jmol image from your frequency file
optimised NH3BH3 molecule |
Questions
E(NH3)= -56.55777 au
E(BH3)= -26.61532 au
E(NH3BH3)= -83.22469 au
Dissociation energy (ΔE) = [E(NH3)+E(BH3)]-E(NH3BH3)= 0.0516 au 0.0516 = 129 kJ/mol
Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?
If one compares C-N bond with B-N then comparatively B-N has a weaker bond (C-N has a bond dissociation of 290kJ/mol). (Reference: https://opentextbc.ca/chemistry/chapter/7-5-strengths-of-ionic-and-covalent-bonds/)
Smf115 (talk) 22:44, 16 May 2018 (BST)Correct calculation method however, an error seems to have been made converting in to kJ/mol
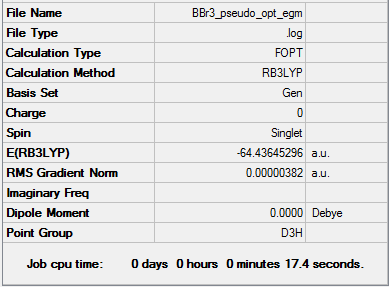
BBr3
Calculation method = RB3LYP
Basis = GEN
Summary table for optimised molecule
"Item" table for optimised molecule
Link to frequency log file and low frequency lines table
Media:BBr3_pseudo_freq_egm.log
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
jmol image from your frequency file
optimised BBr3 molecule |
Vibrational spectrum for BBr3
unique identifier: DOI:10042/202303
Mini project
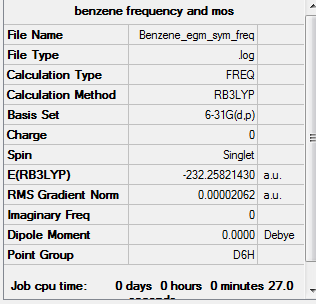
Benzene
Calculation method = RB3LYP
Basis = 6-31G(d.p)
Summary table for optimised molecule
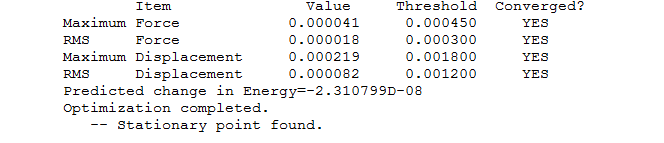
"Item" table for optimised molecule
Link to frequency log file and low frequency lines table
Low frequencies --- -0.0087 -0.0041 -0.0040 12.5838 12.5838 16.4940 Low frequencies --- 414.3526 414.3526 621.2606
jmol image from your frequency file
optimised benzene molecule |
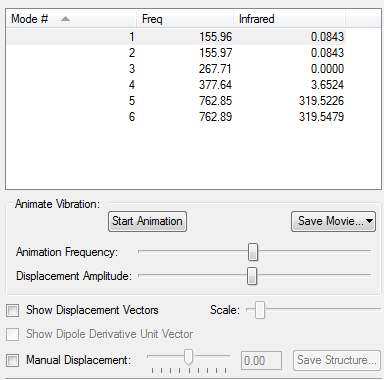
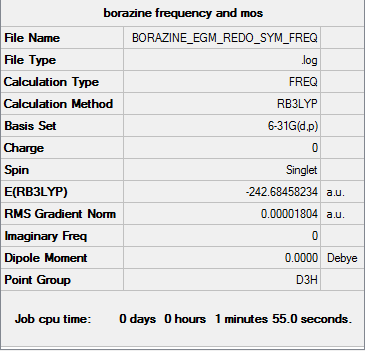
Borazine
Calculation method = RB3LYP
Basis = 6-31G(d.p)
Summary table for optimised molecule
"Item" table for optimised molecule
Link to frequency log file and low frequency lines table
Low frequencies --- -12.6682 -12.4919 -8.6666 -0.0102 -0.0081 0.0803 Low frequencies --- 289.1561 289.1648 404.0196
Jmol image from your frequency file
optimised borazine molecule |
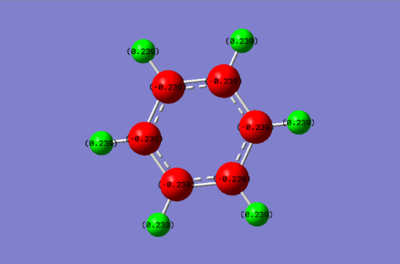
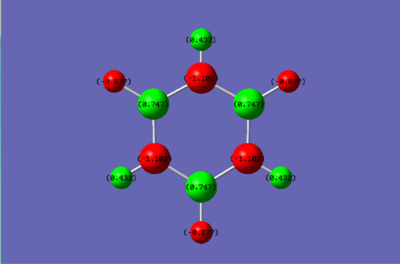
Comparison of charges between Benzene and Borazine
Looking at the charge distribution of benzene, all the charges of the Carbons are identical as well as those of the Hydrogens. Carbon is slightly more electronegative than hydrogen which is why it has the negative charge. The Borazine molecule on the other hand has electronegative nitrogen atoms and electropositive boron atoms. This explains the negative charge of Nitrogen, the positive charge of Boron and the complementary charges of the H atoms.
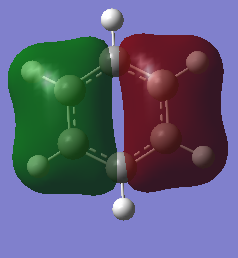
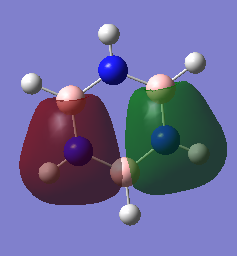
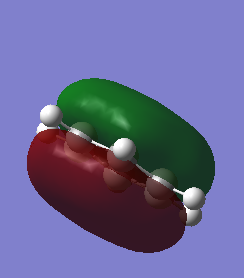
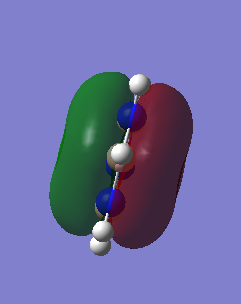
Comparison of three of the same MOs from benzene and Borazine
Aromaticity
Aromaticity is a greater stability of a cyclic structure, and occurs due to a ring of delocalised electrons around the top and bottom of the cyclic structure. The delocalisation renders the structure particularly stable. Another property of aromaticity is the bond lengths within the cyclic structure being the same length, a length in-between the single and double bond lengths.
In an aromatic system there are a number of prerequisites for the presence of aromaticity. As well as the overlapping Pz atomic orbitals, the structure needs to be cycling, and have a pi bond all around the cyclic structure. The aromatic structure must also follow Huckel’s Rule. The Huckel’s rule states that there need to be 4n+2 pi electrons for a cyclic structure to be aromatic and to have that extra stability. If there are 4n electrons then the structure is anti-aromatic - so it shows particular destabilisation - and if it has neither of these values then it isn't aromatic. Lastly, all electrons occupy all the bonding MOs and leave the antibonding and non-bonding MOs unfilled.
There is also the existence of sigma aromaticity, and it appears in saturated cyclic compounds. This is applicable more to inorganic compounds rather than organic ones. The sigma orbitals mirror the symmetries of the Huckel rule with the pi orbitals, so the sigma aromaticity also follows the 4n+2 rule. In saturated cyclic compounds, there is an alternating aromatic and antiaromatic system, and this is due to the sigma aromaticity
The real MOs relate to common conceptions of aromaticity on a fundamental level. Looking at the MO17 it resembles perfectly the delocalised pi system. So aromaticity in principle only focuses on the molecular orbital in which the Pz orbitals are perfectly aligned and overlapped. The other orbitals are less relevant and less of a contributing factor to aromaticity.
(Reference: https://pubs.acs.org/doi/pdf/10.1021/jp048541o)
Smf115 (talk) 22:42, 16 May 2018 (BST)Nice discussion of the key concepts of aromaticity and mention of sigma-aromaticity. The final paragraph isn't wholly correct and it should have been noticed that MO 17 is just one MO of many which contributes towards the delocalisation.
Smf115 (talk) 22:42, 16 May 2018 (BST)Overall a good report however, some issues with missing log files throughout and discussion in the project section missed some of the key points needed.