Year 2 Computational Lab Sam Young
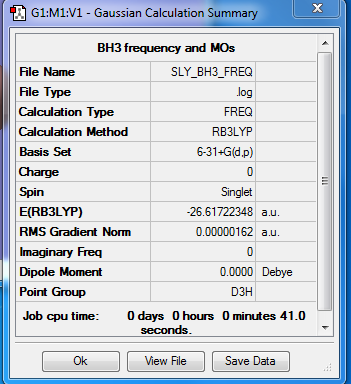
BH3 Molecule
Basis set
6-31G(d,p)
Summary
Item Table
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000013 0.001800 YES
RMS Displacement 0.000006 0.001200 YES
Predicted change in Energy=-6.245437D-11
Optimization completed.
-- Stationary point found.
Link to file
Frequency Table
Full mass-weighted force constant matrix: Low frequencies --- -0.8713 -0.6455 -0.0055 8.0386 12.6754 12.7053 Low frequencies --- 1157.7354 1205.3451 1205.3474
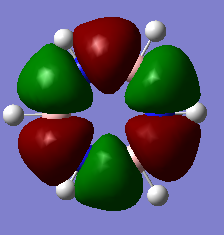
Jmol Image
Optimised BH3 Molecule |
Vibrational Spectrum of BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1157 | 94 | A2" | yes | out-of-plane bend |
| 1205 | 14 | E' | slight | bend |
| 1205 | 14 | E' | slight | bend |
| 2576 | 0 | A1' | no | symmetric stretch |
| 2704 | 143 | E' | yes | asymmetric stretch |
| 2704 | 143 | E' | yes | asymmetric stretch |
There are fewer than 6 peaks present in the IR spectrum despite there being 6 vibrational modes, due to one mode not being IR active, and two pairs of degenerate vibrational modes.
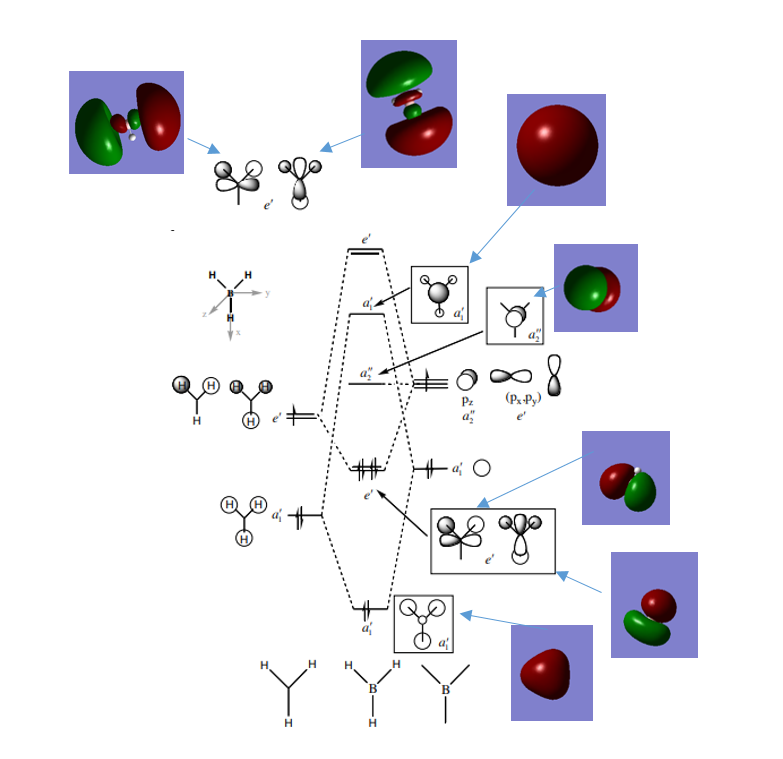
MO Diagram
The qualitative approach provides a simple yet similar image to the computer generated MOs, and in this particular example there are no significant differences between the two. However, when going onto larger molecules, qualitative MOs which get more complicated are often simplified, therefore computer generated MOs are more useful for larger molecules.
Reference: http://www.huntresearchgroup.org.uk/teaching/teaching_comp_lab_year2a/Tut_MO_diagram_BH3.pdf
NH3 Molecule
Basis Set
6-31G(d,p)
Summary
Item
Item Value Threshold Converged?
Maximum Force 0.000110 0.000450 YES
RMS Force 0.000039 0.000300 YES
Maximum Displacement 0.000631 0.001800 YES
RMS Displacement 0.000225 0.001200 YES
Predicted change in Energy=-3.946669D-08
Optimization completed.
-- Stationary point found.
Link to file
Frequency Table
Full mass-weighted force constant matrix: Low frequencies --- -0.2194 -0.1785 -0.0058 20.6664 20.6917 30.8835 Low frequencies --- 1000.9070 1673.7928 1673.7929
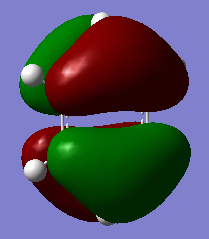
Jmol Image
Optimised NH3 Molecule |
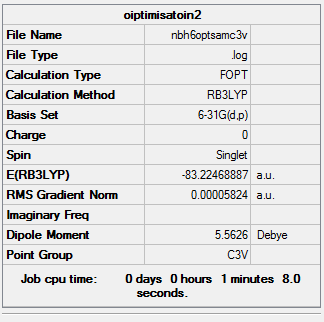
NH3BH3 Molecule
Basis Set
6-31G(d,p)
Summary
Item
Item Value Threshold Converged?
Maximum Force 0.000243 0.000450 YES
RMS Force 0.000054 0.000300 YES
Maximum Displacement 0.001390 0.001800 YES
RMS Displacement 0.000382 0.001200 YES
Predicted change in Energy=-1.938548D-07
Optimization completed.
-- Stationary point found.
Link to file
Frequency Table
Full mass-weighted force constant matrix: Low frequencies --- -0.1897 -0.0663 -0.0074 9.9186 16.5521 16.5669 Low frequencies --- 263.0129 631.3811 638.8655
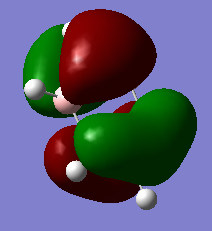
Jmol Image
Optimised NH3BH3 Molecule |
Energies
E(BH3) = -26.61722 a.u.
E(NH3) = -56.56699 a.u.
E(BH3NH3) = -83.22469 a.u.
ΔE=[E(NH3)+E(BH3)] - E(NH3BH3) = 0.04048 a.u.
ΔE = 0.04048/0.0004 = 101 KJ/mol
As a point of comparison the C-N bond has a bond dissociation energy of -290 KJ/mol, suggesting that the value we have calculated for the B-N bond is low, therefore a weaker bond.
Reference: https://opentextbc.ca/chemistry/chapter/7-5-strengths-of-ionic-and-covalent-bonds/
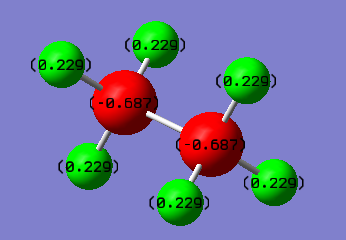
Ethane & BH3NH3
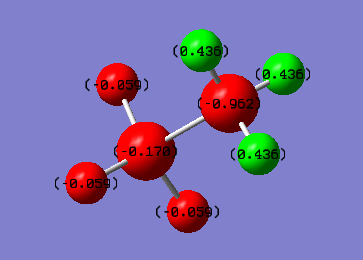
Charge Distribution of Ethane
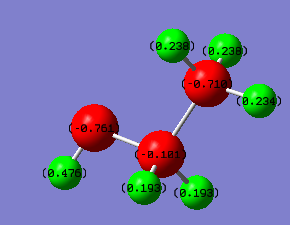
Charge Distribution of BH3NH3
We can clearly see that the C-C Bond for ethane is non polarised due to it being a completely symmetric bond, while the N-B bond is highly polarised, with electron density towards the N, due to a fairly large electronegativity difference between the two atoms. It is possible to chemically manipulate the charges on the bonds by adding an electronegative substituent, for example, an alcohol group.
Charge Distribution of Ethanol
Here the charge distribution along the C-C bond has been altered due to the electronegative oxygen group being added. Now the C bonded to the O has a less negative value due to O drawing electorn density towards itself.
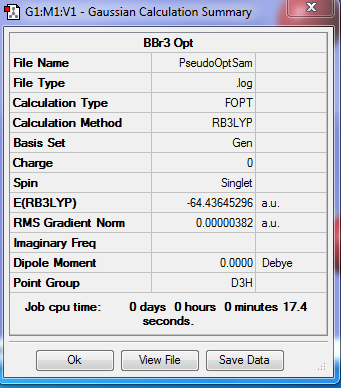
BBr3
Basis Set
GEN
Summary
Item
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.027373D-10
Optimization completed.
-- Stationary point found.
Link to file
Frequency Table
Full mass-weighted force constant matrix: Low frequencies --- -0.0137 -0.0064 -0.0047 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
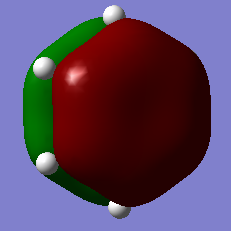
Jmol Image
Optimised BBr3 Molecule |
Aromaticity Mini Project
Benzene
Basis set
6-31G(d,p)
Summary
Item
Item Value Threshold Converged?
Maximum Force 0.000193 0.000450 YES
RMS Force 0.000079 0.000300 YES
Maximum Displacement 0.000830 0.001800 YES
RMS Displacement 0.000294 0.001200 YES
Predicted change in Energy=-4.437691D-07
Optimization completed.
-- Stationary point found.
Link to file
Frequency Table
Full mass-weighted force constant matrix: Low frequencies --- -3.5606 -3.5606 -0.0088 -0.0040 -0.0040 10.0905 Low frequencies --- 413.9582 413.9582 621.1416
Jmol Image
Optimised Benzene Molecule |
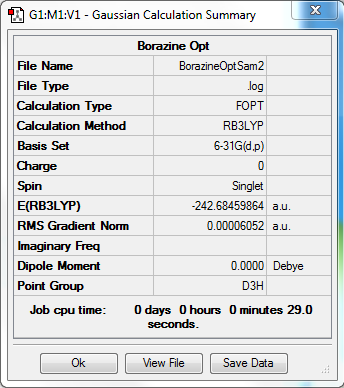
Borazine
Basis Set
6-31G(d,p)
Summary
Item
Item Value Threshold Converged?
Maximum Force 0.000081 0.000450 YES
RMS Force 0.000031 0.000300 YES
Maximum Displacement 0.000238 0.001800 YES
RMS Displacement 0.000071 0.001200 YES
Predicted change in Energy=-8.399586D-08
Optimization completed.
-- Stationary point found.
Link to file
Frequency Table
Full mass-weighted force constant matrix: Low frequencies --- -6.6262 -6.4470 -5.9957 -0.0097 0.0573 0.1401 Low frequencies --- 289.2549 289.2653 403.8557
Jmol Image
Optimised Borazine Molecule |
Charge Distribution
Charge distribution of Benzene
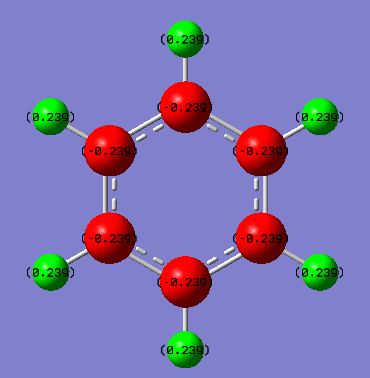
Charge distribution of Borazine
| Atom | Charge distribution | Discuss |
| Benzene - C | -0.239 | All carbon atoms in benzene are identical in terms of charge distributrion due to them all being in the same chemical environments. Negative value as they are more electronegative than the hydrogen atoms, therefore attract electron density. (C = 2.55, H = 2.20) |
| Benzene - H | 0.239 | All Hydrogen atoms in benzene are also identical therefore all have equal charge density. Positive value as they are more electropositive than Carbon so electron density is pulled away from them. (C = 2.55, H = 2.20) |
| Borazine - N | -1.102 | Negative value due to high electronegativity of Nitrogen, attracting electron density towards itself. (N = 3.04) |
| Borazine - B | .0747 | Positive value due to boron being the most electropositive atom in the ring, therefore attracting the least electron density. (B = 2.04) |
| Borazine - H-(N) | 0.432 | Slightly positive value due to electronegativity difference of H and the directly attatched N atom which draws electron density away from the proton. (H = 2.20, N = 3.04) |
| Borazine - H-(B) | -0.077 | Slightly negative due to H being more electronegative than B, therefore it draws electron density towards itself. (B = 2.04, H = 2.20) |
The main difference between the charge distribution of Benzene and Borazine, is that benzene is highly symmetric so all C atoms have the same charge density, as do all H atoms. However, due to the alternating N and B atoms in the Borazine ring, the charge distribution causes the H atoms to have different charge densities depending on whether they're bonded to N or B.
Reference for values : https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/
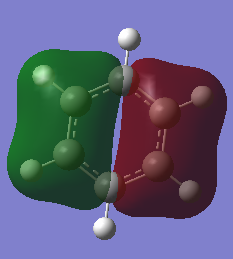
Molecular Orbital Comparison
Ng611 (talk) 23:03, 15 May 2018 (BST) Comparison of the orbitals is correct but some more detail in this section would have been useful. Why are there differences between the benzene/borazine MOs? Which orbitals give rise to the MO's and what is its symmetry?
Concepts of Aromaticity
Aromaticity refers to the additional stabilisatoin of certain molecules, as a result of resonance energy caused by an orthogonal cyclic array of p-orbitals. Furthermore, there are a set of requirements a molecule must fulfill in its ground state in order to be considered as aromatic; i) They must be cyclic with bonds of equal lengths. ii) They must obey Huckels Law i.e. The molecule must have (4n+2) pi electrons in a continuous ring of p-orbitals adjacent to the molecule. (Where n = 0 or n = a positive integer). Although they often are planar molecules, this is not required for aromaticity.
Ng611 (talk) 23:05, 15 May 2018 (BST) What are some of the other phenomena that give rise to aromaticity in non planar molecules?
As well as the extra stabilization gained, one can identify aromaticity by proton-NMR analysis, as in an applied magnetic field, the pi electron ring is induced causing the protons on the outside of the ring to be deshielded, therefore are found downfield, and the reverse would theoretically apply for internal ring protons. (e.g. Benzene gives a singlet at 7.27ppm). Moreover, the concept of antiaromaticity applies for molecules with the same requirements as above, however on require (4n) pi-electrons, and this gives an overall destabilisation, although moleucules often to adopt different conformations to avoid this. Also, sigma aromaticity exists for inorganic saturated rings, whereby these rings display similar characteristics and behavior to pi-aromatic rings.
Ng611 (talk) 23:05, 15 May 2018 (BST) Excellent paragraph!
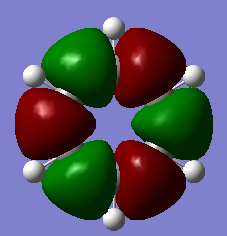
In comparing the real MOs of Benzene to the common concept of aromaticity, it appears as though it is only MO17 which displays the idea of the continuous ring of orthogonal p-orbitals, therefore the real picture suggests that the sigma framework and other pi-bonding orbitals play an important role in the stabilization of aromatic molecule. Due to the fact that the traditional idea of the aromatic ring only contributes to 1 of the 14 filled valence MOs, it can be said that this is a very basic concept and the stabilisation is due to a variety of MOs rather than just the one.
Ng611 (talk) 23:05, 15 May 2018 (BST) True. The nature of aromaticity has been considered by other scientists extensively. Discussing their conclusions would have strengthened this final paragraph significantly.
Ng611 (talk) 23:07, 15 May 2018 (BST) A good report overall. Your MO analysis was good, but your MOs for the 2 highest energy antibonding orbitals were incorrect. The section on aromaticity was generally good - remember to use the same colour scale when comparing charge distributions. Your section on benzene/borazine MO analysis was also good - a rationalisation of the differences between the orbitals would have improved this further, as would a more sophisticated discussion of the contemporary view of aromaticity.
MO17
Reference : https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.200700250
Reference : https://www.ncbi.nlm.nih.gov/pubmed/16839038