Yc8717CYY
BH3 Molecule
1.optimisation with 3-21G basis set

BH3 molecule |
| Molecule name | BH3 |
|---|---|
| B-H bond length | 1.195Å |
| H-H bond angle | 120.0° |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Final energy E(RB3LYP) | -26.4622371(a.u.) |
| Point Group | CS |
Item Value Threshold Converged?
Maximum Force 0.000217 0.000450 YES
RMS Force 0.000105 0.000300 YES
Maximum Displacement 0.000692 0.001800 YES
RMS Displacement 0.000441 0.001200 YES
Predicted change in Energy=-1.635268D-07
Optimization completed.
-- Stationary point found.
2.optimisation with better basis set of 6-31GP

BH3 molecule |
| Molecule name | BH3 |
|---|---|
| B-H bond length | 1.192Å |
| H-H bond angle | 120.0° |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Final energy E(RB3LYP) | -26.61532364(a.u.) |
| Point Group | D3h |
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000015 0.001200 YES
Predicted change in Energy=-1.859017D-10
Optimization completed.
-- Stationary point found.
3.frequency analysis of BH3 molecule
BH3 molecule |
Low frequencies --- -2.2126 -1.0751 -0.0055 2.2359 10.2633 10.3194
Low frequencies --- 1162.9860 1213.1757 1213.1784
Diagonal vibrational polarizability:
0.7180909 0.7179908 1.8414385
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A2" E' E'
Frequencies -- 1162.9860 1213.1757 1213.1784
Red. masses -- 1.2531 1.1072 1.1072
Frc consts -- 0.9986 0.9601 0.9601
IR Inten -- 92.5493 14.0546 14.0583
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.16 0.00 0.10 0.00 -0.10 0.00 0.00
2 1 0.00 0.00 -0.57 0.00 0.08 0.00 0.81 0.00 0.00
3 1 0.00 0.00 -0.57 -0.39 -0.59 0.00 0.14 0.39 0.00
4 1 0.00 0.00 -0.57 0.39 -0.59 0.00 0.14 -0.39 0.00
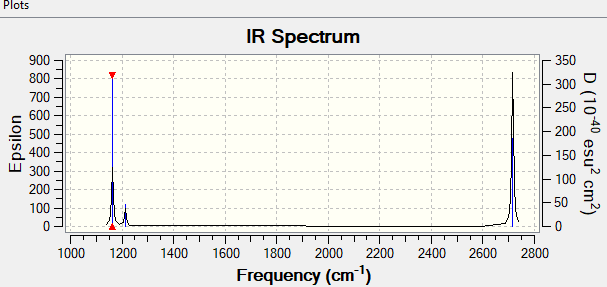
The file of frequency is linked to here
4. additional specific information from your BH3 molecule
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163 | 93 | A1 | yes | out-of-plane bend |
| 1213 | 14 | E | very slight | bend |
| 1213 | 14 | E | very slight | bend |
| 2582 | 0 | A1 | no | symmetric stretch |
| 2716 | 126 | E | yes | asymmetric stretch |
| 3543 | 1 | E | yes | asymmetric stretch |
Spectrum explanation: There are five IR-active frequencies with a change in dipole moment.The one with 2582 cm-1 is IR-inactive with no change in dipole moment, thus it does not show on the spectrum. First three peaks are shown on the left part of the spectrum and the last two are shown on the right. The peal with low intensity may due to small change in dipole moment.
Ng611 (talk) 11:27, 15 May 2019 (BST) You've explained why we don't see one peak, but what about the others?
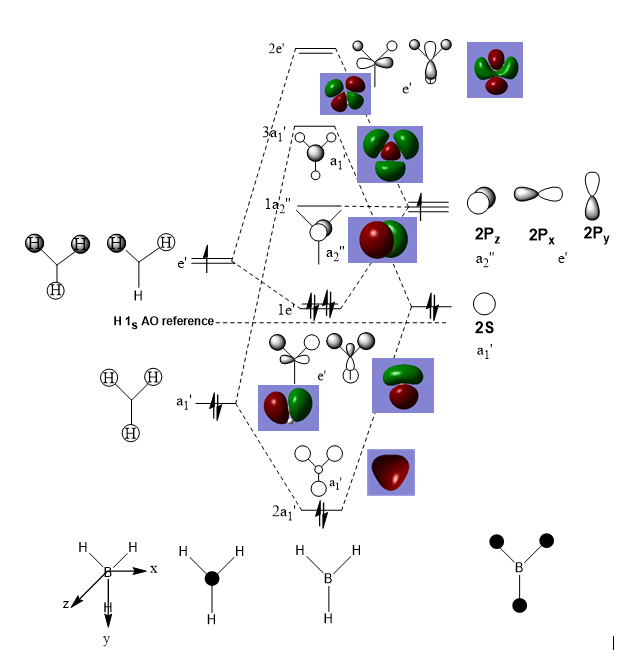
5. MO diagram analysis of BH3 molecule
The MO diagram is shown above. The real MOs are almost the same as predicted LCAO MOs. The only difference is that the real MOs combine the same phases together, producing a cloud. Therefore, the accuracy and usefulness of qualitative MO theory can be believed.
Ammonia-Borane association energies
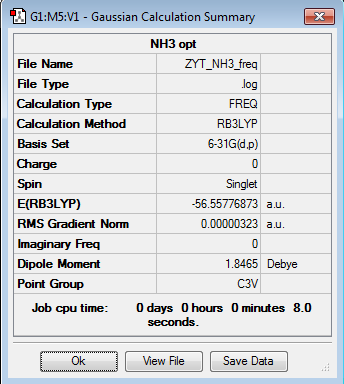
1.NH3 molecule analysis
Low frequencies --- -8.5646 -8.5588 -0.0041 0.0455 0.1784 26.4183
Low frequencies --- 1089.7603 1694.1865 1694.1865
Diagonal vibrational polarizability:
0.1276536 0.1276543 3.2977160
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A E E
Frequencies -- 1089.7603 1694.1865 1694.1865
Red. masses -- 1.1800 1.0644 1.0644
Frc consts -- 0.8256 1.8001 1.8001
IR Inten -- 145.4214 13.5548 13.5549
Atom AN X Y Z X Y Z X Y Z
1 7 0.00 0.00 0.12 -0.07 0.00 0.00 0.00 0.07 0.00
2 1 0.00 -0.21 -0.53 0.76 0.00 0.00 0.00 0.15 0.26
3 1 0.18 0.11 -0.53 0.08 -0.39 0.22 0.39 -0.53 -0.13
4 1 -0.18 0.11 -0.53 0.08 0.39 -0.22 -0.39 -0.53 -0.13
Ng611 (talk) 11:28, 15 May 2019 (BST) Where are your log files and jmol images???
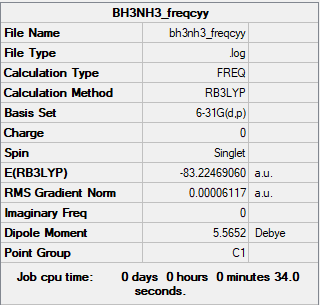
2.NH3BH3 molecule analysis
E(NH3)=-56.55777(a.u.)
E(BH3)=-26.61532(a.u.)
E(NH3BH3)=-83.22469(a.u.)
ΔE=E(NH3BH3) -[E(NH3) +E(BH3)]=-0.0516(a.u.)=-135 kJ/mol
The strength of B-N dative bond is medium according to the energy of -135 kJ/mol calculated above. The dative bond is formed by the donation of Nitrogen lone pair to the vacant p-orbital of boron. The energy is compared to the bond energy of B-O bond (536 kJ/mol) since oxygen is next to N in the periodic table and B-H bond (389 kJ/mol) .
Ng611 (talk) 11:29, 15 May 2019 (BST) Cite where you got your bond enthalpy values from.
Low frequencies --- -0.0005 -0.0002 0.0014 17.6705 28.0332 40.2175
Low frequencies --- 266.5190 632.3638 639.4914
Diagonal vibrational polarizability:
2.5462308 2.5487470 5.0153318
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 266.5159 632.3635 639.4913
Red. masses -- 1.0078 4.9944 1.0451
Frc consts -- 0.0422 1.1767 0.2518
IR Inten -- 0.0000 13.9812 3.5410
NI3 Molecule
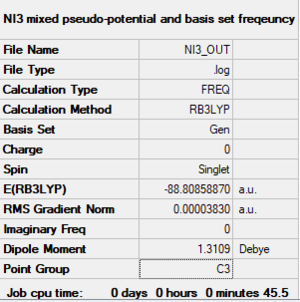
NI3 molecule |
The file of frequency is linked to here Optimised N-I distance=2.184 Å
Item Value Threshold Converged?
Maximum Force 0.000029 0.000450 YES
RMS Force 0.000020 0.000300 YES
Maximum Displacement 0.000228 0.001800 YES
RMS Displacement 0.000138 0.001200 YES
Predicted change in Energy=-6.786309D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -12.7477 -12.7416 -6.4258 -0.0140 0.0210 0.0991
Low frequencies --- 101.0290 101.0295 147.4197
Diagonal vibrational polarizability:
12.5287306 12.5312034 1.3363968
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
E E A
Frequencies -- 101.0290 101.0295 147.4197
Red. masses -- 115.8383 115.8394 103.1309
Frc consts -- 0.6966 0.6966 1.3205
IR Inten -- 1.0183 1.0173 0.8959
Project section: Ionic liquids (designer solvents)
1.Analysis [N(CH3)4]+ Molecule
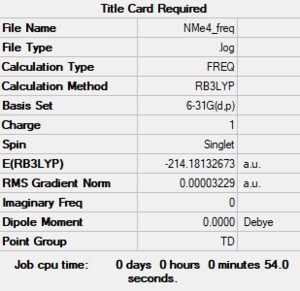
[N(CH)] molecule |
The file of frequency is linked to here
Item Value Threshold Converged?
Maximum Force 0.000020 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000673 0.001800 YES
RMS Displacement 0.000185 0.001200 YES
Predicted change in Energy=-9.527444D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- 0.0008 0.0009 0.0010 34.4140 34.4140 34.4140
Low frequencies --- 216.5368 315.9455 315.9455
Diagonal vibrational polarizability:
1.3863159 1.3863159 1.3863159
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A2 T1 T1
Frequencies -- 216.5368 315.8718 315.8718
Red. masses -- 1.0078 1.0333 1.0333
Frc consts -- 0.0278 0.0607 0.0607
IR Inten -- 0.0000 0.0000 0.0000
2.Analysis [P(CH3)4]+ Molecule

[P(CH)] molecule |
The file of frequency is linked to here
Item Value Threshold Converged?
Maximum Force 0.000078 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.000447 0.001800 YES
RMS Displacement 0.000145 0.001200 YES
Predicted change in Energy=-1.691140D-07
Optimization completed.
-- Stationary point found.
Low frequencies --- -22.4575 -13.2993 -0.0035 -0.0024 0.0009 22.5793
Low frequencies --- 154.7484 184.8269 191.1732
Diagonal vibrational polarizability:
3.5428708 3.5467010 3.5385839
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 154.7385 184.8015 191.1162
Red. masses -- 1.0087 1.0257 1.0258
Frc consts -- 0.0142 0.0206 0.0221
IR Inten -- 0.0007 0.0005 0.0007
3.Charge analysis of [N(CH3)4]+ and [P(CH3)4]+ Molecule

| Type of element | charge (C) |
| Nitrogen | -0.295 |
| Carbon | -0.483 |
| Hydrogen | +0.269 |
| Type of element | charge (C) |
| phosphorous | +1.668 |
| Carbon | -1.060 |
| Hydrogen | +0.298 |
Comparison of their charge distributions
The charge distribution is depends on the charge density on an atom which is composed of individual charge particle and are separated by regions. The charge density around an atom is related to the electronegativity of that element.
Nitrogen (Xp=3.04) is more electronegative then carbon (Xp=2.55) , thus N would pull electrons towards itself. However, carbon is a lot more electronegative than hydrogen and there are three hydrogens around it so this may be the reason carbon shows a more negative value for charge than N.
Phosphorous (Xp=2.19) is less electronegative than carbon (Xp=2.55), leading to a positive charge on P and a negative charge on C. Carbon in [P(CH3)4]+ molecule is more negative than in [N(CH3)4]+ molecule is because it is more electronegative than both H and P, pulling electrons from P and C towards itself;while in [N(CH3)4]+, some electrons of C are pulled away by N.
Ng611 (talk) 11:33, 15 May 2019 (BST) What about the effect of symmetry on charge distribution?
Analysis of charge on N in[NR4]+ molecules (R=alkyl)
The formal positive charge on N means the formal charge on it is +1 and the cation is formed by taking an electron from N according to the traditional description of this type of molecule. However, the charge value of N as shown in the table above is -0.295, which means that the positive charge is not located on N. The positive charge is actually located on the hydrogens due to the poor electronegativity (Xp=2.2), so its electron is pulled away towards N.
Ng611 (talk) 11:33, 15 May 2019 (BST) Yes but why does formal electron counting give this result? More detail is needed.
2.Analysis of [N(CH3)4]+ occupied MOs
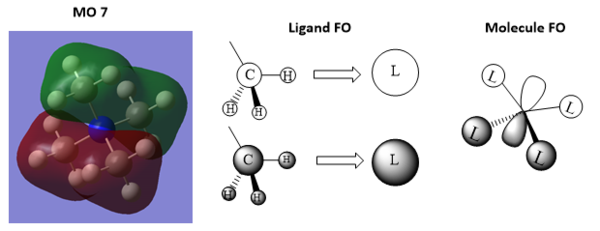

Ng611 (talk) 11:36, 15 May 2019 (BST) Your FO is incorrect for MO10. You need something more like the FO for MO7

Ng611 (talk) 11:37, 15 May 2019 (BST) You should annotate your diagrams with the key orbital interactions.