Y2complabhaien910
BH3
Summary
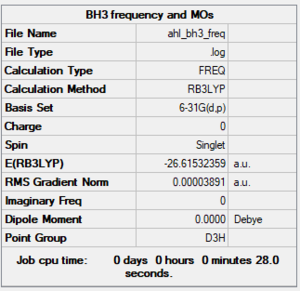
Method and Basis Set: RB3LYP/6-31G (d,p)
Optimisation
Item Value Threshold Converged? Maximum Force 0.000078 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000306 0.001800 YES RMS Displacement 0.000153 0.001200 YES
Link: AHL BH3 FREQ.LOG
Frequency Analysis
Low frequencies --- -28.3881 -26.4571 -26.4538 -0.0055 0.1739 0.4193 Low frequencies --- 1162.7284 1213.0022 1213.0049
3D Image of BH3
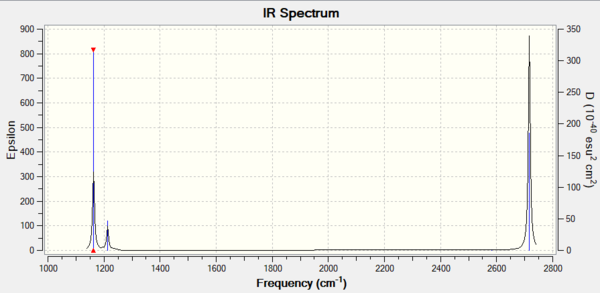
Vibrational Spectrum of Optimised BH3
| Mode | Frequency (cm-1) | Infrared (Intensity) | Symmetry | IR Activity | Vibration Type |
|---|---|---|---|---|---|
| 1 | 1163 | 93 | a2" | Yes | Out of plant wagging |
| 2 | 1213 | 14 | e' | Slight | In plane scissoring |
| 3 | 1213 | 14 | e' | Slight | In plane rocking |
| 4 | 2582 | 0 | a1' | No | Symmetric stretching |
| 5 | 2716 | 126 | e' | Yes | Asymmetric stretching |
| 6 | 2716 | 126 | e' | Yes | Asymmetric stretching |
Ng611 (talk) 21:26, 5 June 2019 (BST) Your vibrations at 1213 cm-1 are degenerate and therefore should have the same assignment.
Only 3 IR active signals are seen in the IR spectrum. Among the 6 vibrational frequencies calculated by Gaussian, vibrational modes 2 and 3, 5 and 6 are degenerate as their stretching frequencies are equivalent between each other. Vibrational mode 4 is symmetric so there is no overall dipole moment, hence it is not IR active.
MO Diagram of Optimised BH3
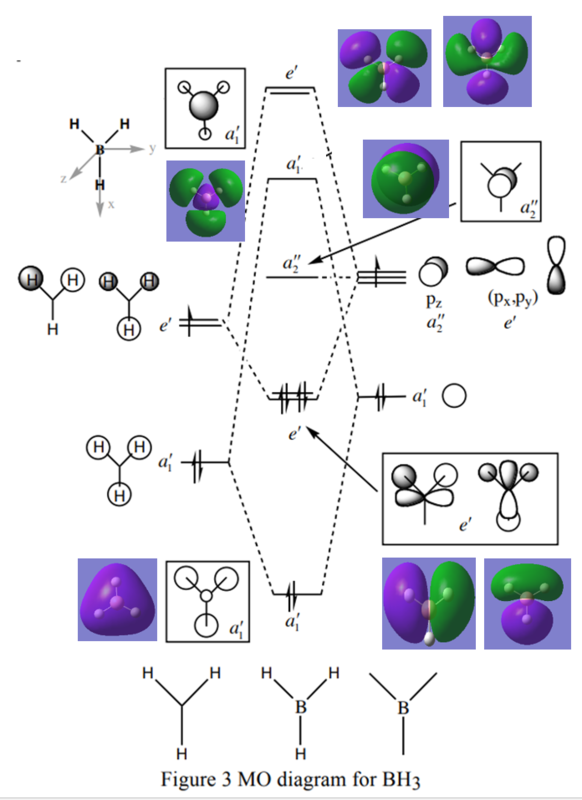
The Linear Combination of Atomic Orbitals (LCAO) MOs are slightly different to the Hartree-Fock (real) MOs because LCAO only provides an approximation to the exact electronic structure of molecules. MO theory is useful in predicting energy levels and shapes of the MOs. The major difference is that Hartree-Fock is constructed from distorted AOs due to electronic distortions from ionic or covalent contributions. [2]
Ng611 (talk) 21:30, 5 June 2019 (BST) Keep in mind that you're not using Hartree-Fock in this lab, you're using DFT. Also remember that none of these methods are 'real' in the sense that they're not exact solutions to the Schrodinger Eqn. Several so-called 'post Hartree-Fock' methods are (in theory but not in practice) full solutions to the Schrodinger Eqn.
NH3
Summary
Method and Basis Set: RB3LYP/6-31G (d,p)

Optimisation
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000040 0.001800 YES
RMS Displacement 0.000013 0.001200 YES
Link: NH3FREQUENCYANALYSIS.LOG
Frequency Analysis
Low frequencies --- -8.5223 -8.4750 -0.0029 0.0335 0.1918 26.4067 Low frequencies --- 1089.7616 1694.1862 1694.1866
3D Image of NH3
NH3BH3
Summary
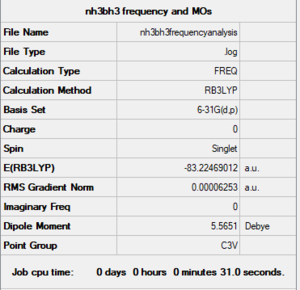
Method and Basis Set: RB3LYP/6-31G (d,p)
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000113 0.000450 YES
RMS Force 0.000063 0.000300 YES
Maximum Displacement 0.000610 0.001800 YES
RMS Displacement 0.000350 0.001200 YES
Link: NH3BH3FREQUENCYANALYSIS.LOG
Frequency Analysis
Low frequencies --- -0.0618 -0.0459 -0.0067 21.6073 21.6132 40.3723 Low frequencies --- 265.9940 632.3731 640.1266
3D Image of NH3BH3
Association Energies: Ammonia-Borane, NH3BH3
Calculations
From the summary tables of NH3 and NH3BH3 shown above:
E(NH3) = -56.55776863
E(BH3) = -26.61532359
E(NH3BH3) = -83.22469012
ΔE = E(NH3BH3) - [E(BH3) + E(NH3)]
= - 0.0516 a.u. = - 135 kJ/mol (Lit. value [3] = 184 kJ/mol)
Bond energy of B-N = -135 kJ/mol
Bond energy of C-C = -350 kJ/mol [4]
Bond energy of C-H = -410 kJ/mol [5]
The bond energy of B-N is approximately one-third of that of C-C bond. Dative B-N bond is a weak bond.
Although NH3BH3 is isoelectronic and isostructural with ethane, C2H6, the central bond in ammonia borane is a dative bond rather than the covalent C-C bond in ethane. The strong intermolecular dihydrogen can also form between NH3BH3 molecules. Hence, solid and gaseous state NH3BH3 have different B-N bond lengths.
Ng611 (talk) 21:32, 5 June 2019 (BST) Excellent!
NI3
Summary
Method and Basis Set: RB3LYP/GEN
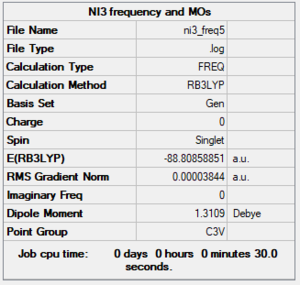
Optimisation
Optimised N-I distance: 2.18362 Å
Ng611 (talk) 21:33, 5 June 2019 (BST) Too many d.p. here. Your values are accurate to about 0.001 A and the accuracy of your reported bond value should account for this.
Item Value Threshold Converged?
Maximum Force 0.000064 0.000450 YES
RMS Force 0.000038 0.000300 YES
Maximum Displacement 0.000488 0.001800 YES
RMS Displacement 0.000278 0.001200 YES
Link: NI3 FREQ5.LOG
Frequency Analysis
Low frequencies --- -12.7380 -12.7319 -6.2907 -0.0039 0.0188 0.0633 Low frequencies --- 101.0326 101.0333 147.4124
3D Image of NI3
Ionic Liquids: Designer Solvent - [N(CH3)4]+
Summary
Method and Basis Set: RB3LYP/6-31G (d,p)
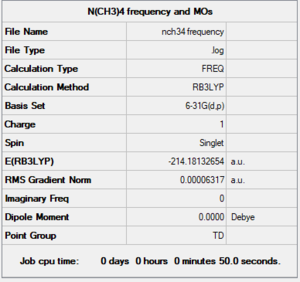
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000108 0.000450 YES
RMS Force 0.000063 0.000300 YES
Maximum Displacement 0.000820 0.001800 YES
RMS Displacement 0.000438 0.001200 YES
Link: NCH34 FREQUENCY.LOG
Frequency Analysis
Low frequencies --- -0.0011 -0.0006 -0.0004 35.2376 35.2376 35.2376 Low frequencies --- 218.5723 317.3185 317.3185
3D Image of [N(CH3)4]+
Charge Distribution of [N(CH3)4]+
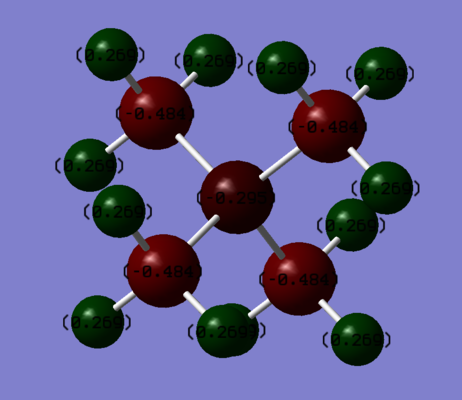
The formal charge is convenient as it associates full charges with certain atoms. They can be derived from the Lewis structure. However, in reality, the charges are delocalised among the atoms in the molecules, and the overall molecule may be neutral. Formal charge is only useful for indicating the charge of the overall molecule, not on the individual atoms.
Ng611 (talk) 21:35, 5 June 2019 (BST) By definition, if the molecule carries an overall charge, it cannot be neutral. Be more precise with your language.
For [N(CH3)4]+, the positive charge is positioned on the nitrogen because it is tetravalent. By using GaussView, it is now possible to develop very accurate descriptions of the electron distributions in molecules. The positive charge resides on the methyl groups, and each group carries [-0.484 + (3 x 0.269)] = 0.323, the overall charge for a CH3 group. N is more electronegative than C, and so N is expected to have the most negative charge. However, despite N is more electronegative, most of the negative charge resides on the C-C bonds due to the donation of lone pairs from N to the methyl groups.
| Charge on Central Heteroatom (N) | Charge on C | Charge on H |
|---|---|---|
| -0.295 | -0.484 | 0.269 |
LCAO-MO Diagram of [N(CH3)4]+
| MO | Energy (a.u.) | MO Diagram | LCAO-MO Diagram |
|---|---|---|---|
| 7 | (-)0.9256 | 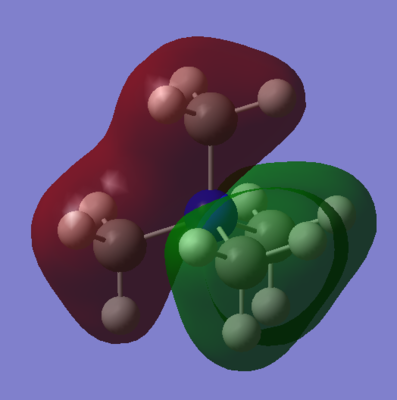 |
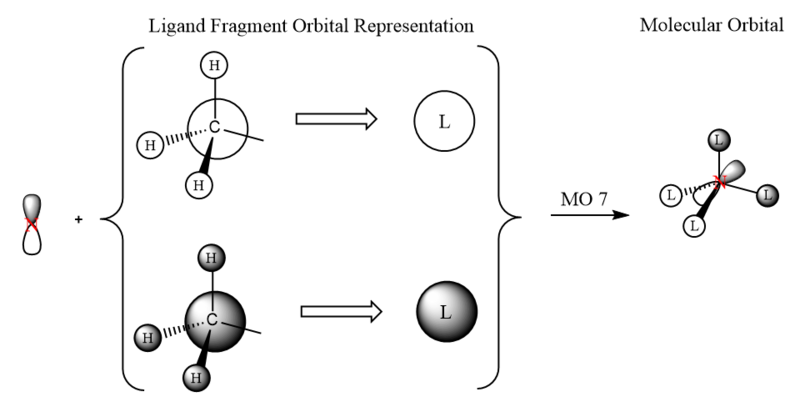 |
| 14 | (-)0.6225 | 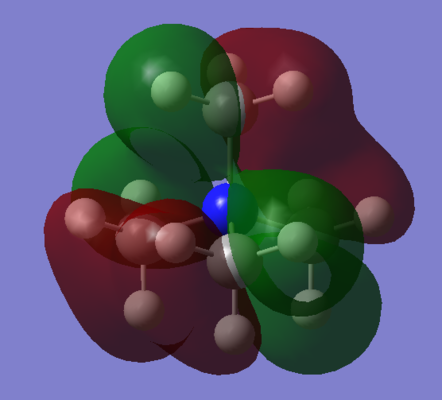 |
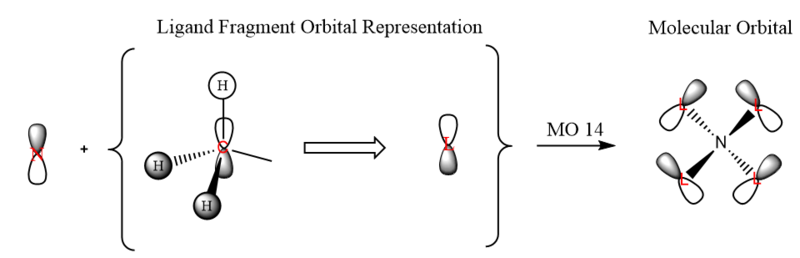 |
| 16 | (-)0.5803 | 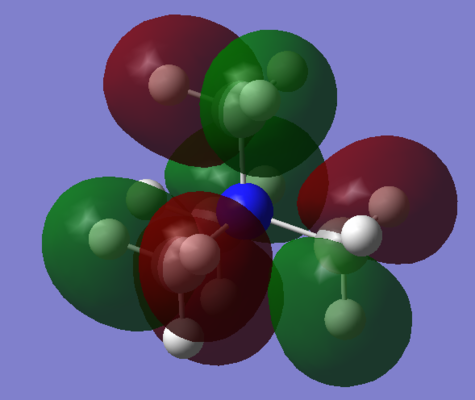 |
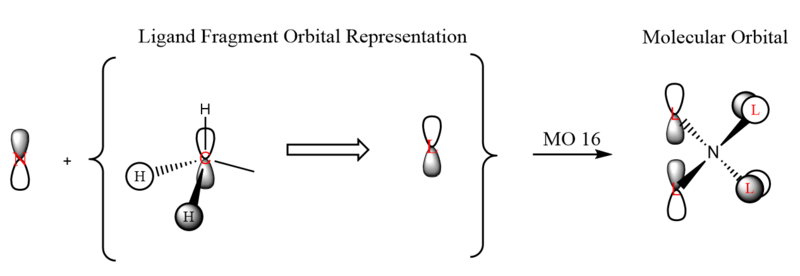 |
Annotated MO Diagrams
| MO | Annotated LCAO-MO Diagram |
|---|---|
| 7 |  |
| 14 | 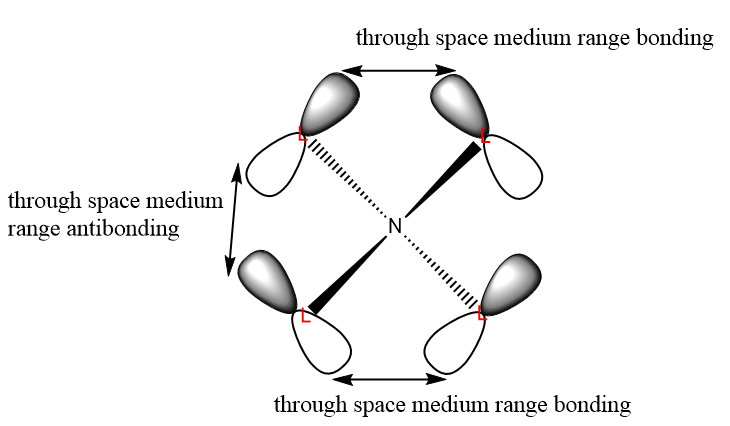 |
| 16 | 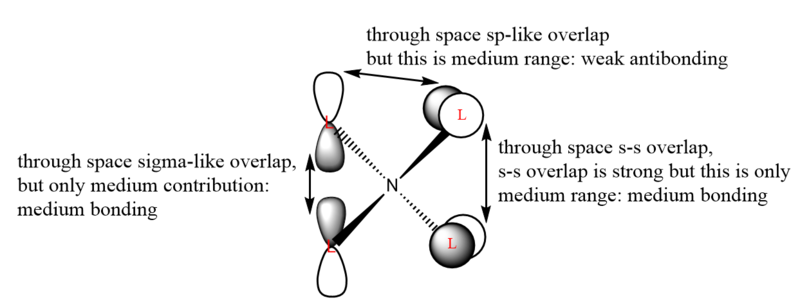 |
Ng611 (talk) 21:37, 5 June 2019 (BST) Good!
Ionic Liquids: Designer Solvent - [P(CH3)4]+
Summary
Method and Basis Set: RB3LYP/6-31G (d,p)
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000187 0.000450 YES
RMS Force 0.000109 0.000300 YES
Maximum Displacement 0.001460 0.001800 YES
RMS Displacement 0.000878 0.001200 YES
Link: PCH34 FREQUENCY.LOG
Frequency Analysis
Low frequencies --- 0.0011 0.0013 0.0027 50.8172 50.8172 50.8172 Low frequencies --- 186.8946 211.7454 211.7454
3D Image of [P(CH3)4]+
Charge Distribution of [P(CH3)4]+

| Charge on Central Heteroatom (P) | Charge on C | Charge on H |
|---|---|---|
| 1.667 | -1.060 | 0.298 |
The charge on central heteroatom P is most positive as compared to C and H because Phosphorus is most electropositive among them.
Bibliography
- ↑ P. Hunt; Molecular Orbitals in Inorganic Chemistry, Hunt Research Group
- ↑ D. Butler, N. Kestner; Comparison of the Hartree-Fock and LCAO-MO Solutions for the Interaction of Rare-Gas Atoms in the Region of Small Overlap, J. Chem. Phy., 1970, 53(5), pp. 1704 - 1707
- ↑ K. Dreux, L. McNamara, J. Kelly; Probing Dative and Dihydrogen Bonding in Ammonia Borane with Electronic Structure Computations and Raman under Nitrogen Spectroscopy, J. Phy. Chem., 2017, pp. 5884 - 5893
- ↑ M. Mitoraj; Bonding in Ammonia Borane: An Analysis Based on the Natural Orbitals for Chemical Valence and the Extended Transition State Method (ETS-NOCV), J. Phys. Chem., 2011, 115(51), pp. 14708 - 14716
- ↑ M. Mitoraj; Bonding in Ammonia Borane: An Analysis Based on the Natural Orbitals for Chemical Valence and the Extended Transition State Method (ETS-NOCV), J. Phys. Chem., 2011, 115(51), pp. 14708 - 14716
