Y2Mod:MG4417
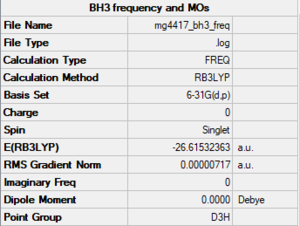
BH3
Method/basis set: B3LYP/6-31G(d,p)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000056 0.001800 YES RMS Displacement 0.000028 0.001200 YES Predicted change in Energy=-1.214268D-09 Low frequencies --- -8.2092 -1.7273 -0.0054 0.6025 6.1863 6.4229 Low frequencies --- 1162.9646 1213.1613 1213.1640
optimised borane molecule |
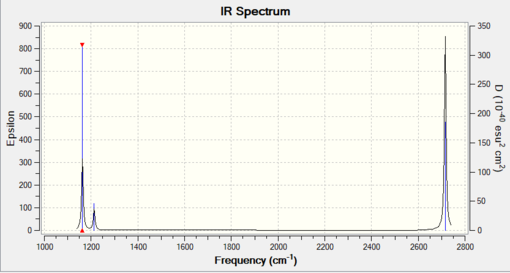
Spectrum and vibrational analysis
| Mode | Frequency | Intensity | Symmetry | Description of vibration |
|---|---|---|---|---|
| 1 | 1163 | 92.6 | A2" | symmetric bend, out of plane |
| 2 | 1213 | 14.1 | E' | symmetric wag |
| 3 | 1213 | 14.1 | E' | asymmetric wag |
| 4 | 2582 | 0 | A1' | symmetric stretch, inactive |
| 5 | 2716 | 126.3 | E' | asymmetric stretch |
| 6 | 2716 | 126.3 | E' | asymmetric stretch |
Why are there less than six peaks in the spectrum?
Modes 2 and 3, as well as modes 5 and 6, are doubly degenerate so appear together on the spectrum. Mode 4 is IR inactive (since there is no change in dipole) so it does not appear at all on the IR spectrum.
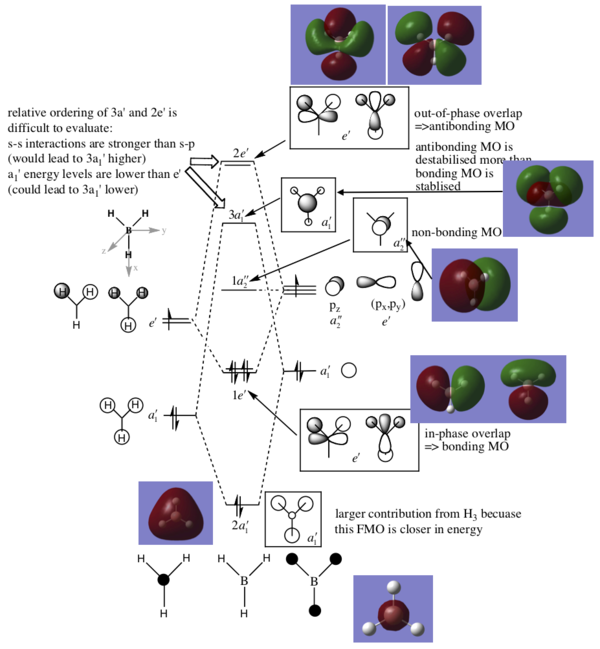
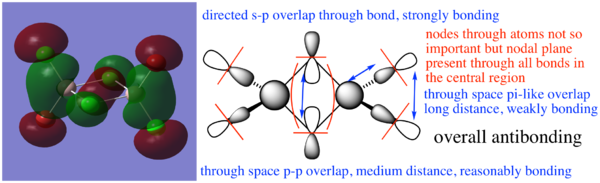
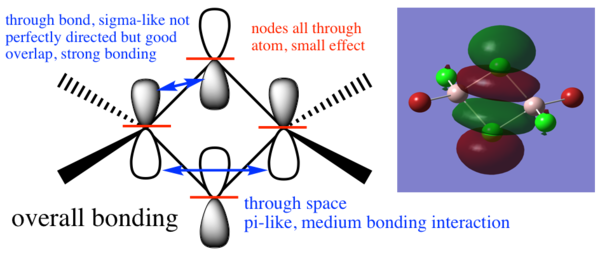
MO diagram
From Hunt Research Group
The LCAO MOs are similar to the real MOs with an obvious difference being the lack of overlap in the LCAO diagrams. The LCAO diagrams make it simple to identify involved orbitals and, with some knowledge of overlapping orbitals, we can easily approximate the electron distribution that would be seen in the real MOs so it is a useful tool. The LCAO orbitals are, of course, not sufficient for precise calculations.
Ng611 (talk) 19:12, 27 May 2019 (BST) Can you identify any differences betwen quantitative and qualitative MO theory?
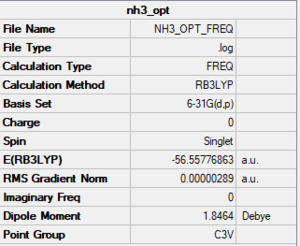
NH3
Method/basis set: B3LYP/6-31G(d,p)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES Predicted change in Energy=-9.075210D-11 Low frequencies --- -11.5223 -11.4866 -0.0029 0.0246 0.1415 25.6160 Low frequencies --- 1089.6618 1694.1735 1694.1738
optimised ammonia molecule |
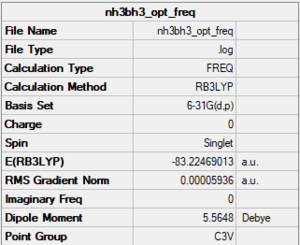
NH3BH3
Method/basis set: B3LYP/6-31G(d,p)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000121 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000569 0.001800 YES RMS Displacement 0.000318 0.001200 YES Predicted change in Energy=-1.716563D-07 Low frequencies --- -0.0618 -0.0459 -0.0066 21.6264 21.6324 40.3000 Low frequencies --- 265.9346 632.2346 640.0641
optimised NH3BH3 molecule |
E(NH3)= -56.55776863
E(BH3)= -26.61532363
E(NH3BH3)= -83.22469013
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
-83.22469013 - (-26.61532363 + -56.55776863) = -0.05159787 a.u.
1 hartree (a.u.) = 2625.5 kJ/mol
-0.05159787 a.u. = -135 kJ/mol (3 s.f.)
Ng611 (talk) 19:14, 27 May 2019 (BST) Good calculation.
The normal bond enthalpy at 298 K of the B-N bond is 389 kJ/mol [ref] so our calculated B-N dative bond is relatively weak.
Ng611 (talk) 19:14, 27 May 2019 (BST) A random .pdf from the internet is not a good reference. Try finding a scientific paper/textbook/databook instead.
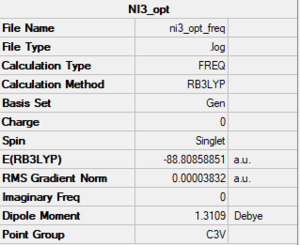
NI3
Method/basis set: B3LYP/GEN (N: 6-31G(d,p); I: LanL2DZ)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000067 0.000450 YES RMS Force 0.000044 0.000300 YES Maximum Displacement 0.000486 0.001800 YES RMS Displacement 0.000363 0.001200 YES Predicted change in Energy=-6.369422D-08 Low frequencies --- -12.7375 -12.7314 -6.2898 -0.0039 0.0188 0.0633 Low frequencies --- 101.0325 101.0332 147.4122
optimised NI3 molecule |
The optimised B-I bond distance was found to be 2.184 Å.
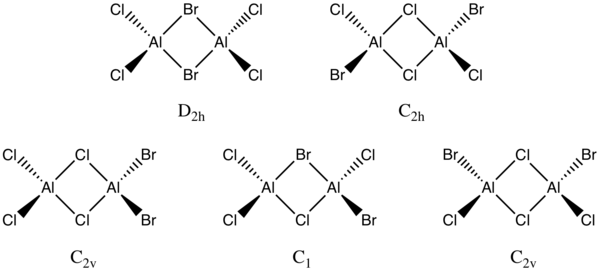
Project: Main Group Halides- Al2Cl4Br2
Isomers of Al2Cl4Br2 with symmetry point groups.
Energy of two isomers
Energy calculations were carried out twice. First with a pseudo-potential on all atoms, this was done because it would make for the most reliable comparison between isomers. It would be unreliable to attribute changes between isomers to only changing position and not to the pseudo-potential. After difficulties in attaining convergence with these calculations, the pseudo-potential was only applied on the heaviest atom, Br. Both tests are included in this work, however only the second can be considered reliable due to problems with convergence in the first.
Pseudo-potential on all atoms
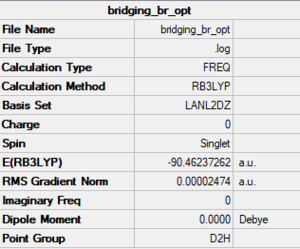
Bridging Br
Method/basis set: B3LYP/LanL2DZ
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000035 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.000585 0.001800 YES RMS Displacement 0.000195 0.001200 YES Predicted change in Energy=-5.296151D-08 Low frequencies --- -3.1172 -1.5998 0.0000 0.0000 0.0000 3.0050 Low frequencies --- 16.6606 52.3978 72.9198
optimised molecule |
Trans terminal Br
Method/basis set: B3LYP/LanL2DZ
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000076 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.001683 0.001800 YES RMS Displacement 0.000526 0.001200 YES Predicted change in Energy=-1.279182D-07 Low frequencies --- -2.6678 -0.0001 0.0000 0.0001 3.1983 3.3077 Low frequencies --- 18.0970 40.4821 64.5629
optimised molecule |
Monomer- AlCl2Br
Method/basis set: B3LYP/LanL2DZ
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000146 0.000450 YES RMS Force 0.000078 0.000300 YES Maximum Displacement 0.001928 0.001800 NO RMS Displacement 0.001635 0.001200 NO Predicted change in Energy=-2.520022D-07 Low frequencies --- 0.0000 0.0000 0.0000 3.6917 4.9979 6.2169 Low frequencies --- 108.1004 119.9123 168.6209
This test may have not converged because using a pseudo-potential on all atoms in this case is not appropriate. Furthermore, it could have been that the LanL2DZ basis set was not accurate enough for this particular calculation.
optimised molecule |
Results
E(Br trans)= -90.47287952 a.u.
E(Br bridging)= -90.46237262 a.u.
E(Br trans) - E(Br bridging)= -0.0105069 a.u. = -27.6 kJ/mol.
Isomer with Br trans is more stable, discussion in following section.
Dissociation energy of this isomer:
2(E(monomer))-E(trans)
2(-45.21900101)-(-90.47287952)= 0.0348775 a.u. = 91.6 kJ/mol.
Positive energy tells us that this isomer is lower in energy than the dissociation products.
Pseudo-potential on Br
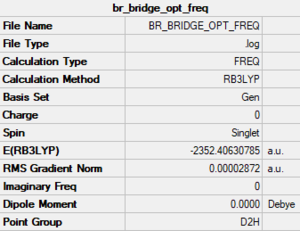
Bridging Br
Method/basis set: B3LYP/GEN (Al, Cl: 6-31G(d,p); Br: LanL2DZ)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000068 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.001356 0.001800 YES RMS Displacement 0.000596 0.001200 YES Predicted change in Energy=-1.340348D-07 Low frequencies --- -5.4042 -5.0662 -3.4974 -0.0040 -0.0036 -0.0027 Low frequencies --- 14.8547 63.2537 86.0200
optimised molecule |
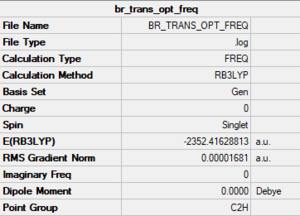
Trans terminal Br
Method/basis set: B3LYP/GEN (Al, Cl: 6-31G(d,p); Br: LanL2DZ)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000025 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000503 0.001800 YES RMS Displacement 0.000212 0.001200 YES Predicted change in Energy=-2.217341D-08 Low frequencies --- -4.2264 -2.1529 -0.0020 0.0022 0.0031 1.1140 Low frequencies --- 17.7223 48.9616 72.9504
optimised molecule |
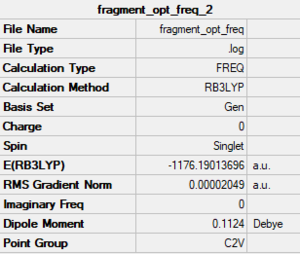
Monomer- AlCl2Br
Method/basis set: B3LYP/GEN (Al, Cl: 6-31G(d,p); Br: LanL2DZ)
Summary table
The LOG file is linked to here
Convergence and low frequency data
Item Value Threshold Converged? Maximum Force 0.000054 0.000450 YES RMS Force 0.000022 0.000300 YES Maximum Displacement 0.000277 0.001800 YES RMS Displacement 0.000129 0.001200 YES Predicted change in Energy=-1.128145D-08 Low frequencies --- -3.1104 -0.0027 0.0012 0.0035 1.9802 2.3808 Low frequencies --- 120.5065 133.8377 185.7407
optimised molecule |
Results
E(Br trans)= -2352.41628813 a.u.
E(Br bridging)= -2352.40630785 a.u.
E(Br trans) - E(Br bridging)= -0.00998028 a.u. = -26.2 kJ/mol.
Ng611 (talk) 19:22, 27 May 2019 (BST) Values using your basis set and XC functional are generally accurate to ~1 kJ/mol so your values should be reported to a similar degree of accuracy.
Isomer with Br trans is more stable. Intuitively, this makes sense since the bromide substituents are large and the trans arrangement maximises the distance between the two Br atoms and minimises repulsion, lowering the energy of the molecule. An arrangement with bridging Cl atoms may be favourable also, perhaps because the chlorine atom is compact and so has better overlap with the Al (similar in radius to Cl) orbitals involved in the central bonding structure. A more stable bond between the Al and the bridging atom is, of course, an important contribution the overall stability.
Ng611 (talk) 19:23, 27 May 2019 (BST) Good calculation and good discussion.
Dissociation energy of this isomer:
2(E(monomer))-E(trans)
2(-1176.19013696)-(-2352.41628813)= 0.03601420999 a.u. = 94.6 kJ/mol.
Positive energy tells us that this isomer is lower in energy than the dissociation products. The dimer completes an octet of electrons around the aluminium centres so this is expected to be more stable. Hyperconjugation of the p orbitals on Al (empty) and the filled p orbitals of Br and Cl does occur in the monomer so the energy is not so high that it is completely unfavourable but dimerisation provides further stabilisation by the donation of lone pairs from the bridging atoms into the aluminium centres and the subsequent formation of new molecular orbitals resulting in stability.
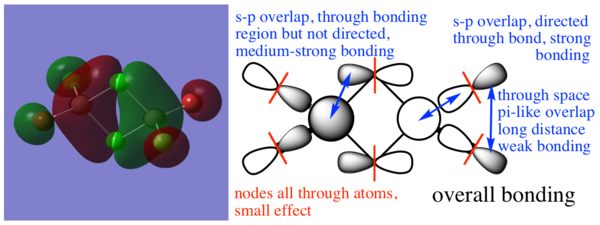
Molecular orbitals of the Br trans isomer
MO 17
MO 21
MO 23