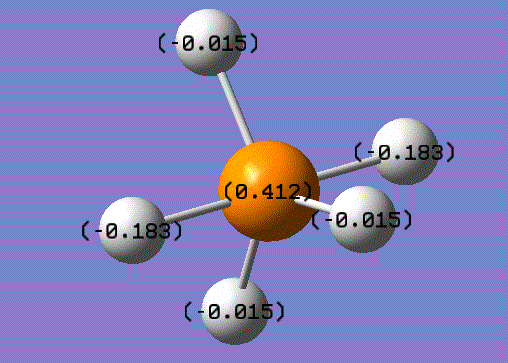Xianqize
Appearance
NH3
Optimisation information
Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -56.55776873 a.u. Point Group = C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986278D-10
Structure
media:XIANQIZE_NH3_OPTF_POP.LOG
test molecule |
Bond length = 1.01798 a.u. Bond angle = 105.74
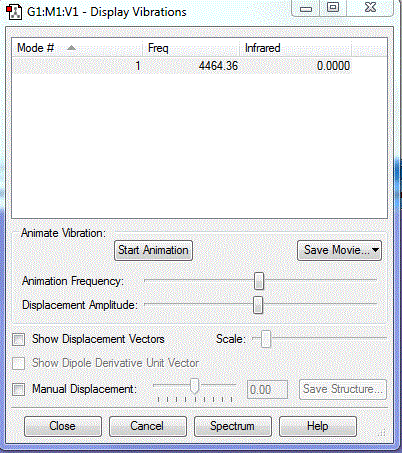
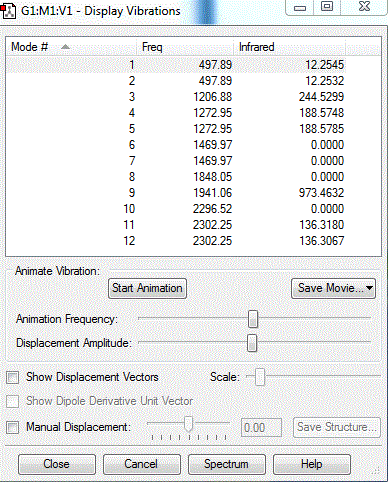
Frequency analysis
how many modes do you expect from the 3N-6 rule?
6
which modes are degenerate (ie have the same energy)?
2&3 and 5&6
which modes are "bending" vibrations and which are "bond stretch" vibrations?
1&2&3 , 4&5&6
which mode is highly symmetric?
4
one mode is known as the "umbrella" mode, which one is this?
1
how many bands would you expect to see in an experimental spectrum of gaseous ammonia?
2
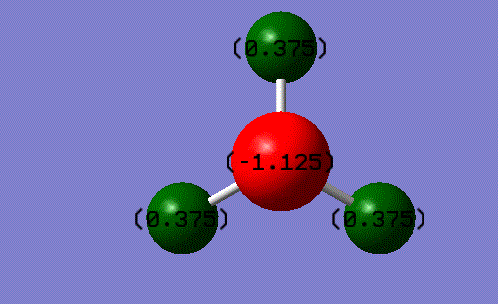
Charge on atoms
charge on the N-atom = -1.125
charge on the H-atom = +0.375
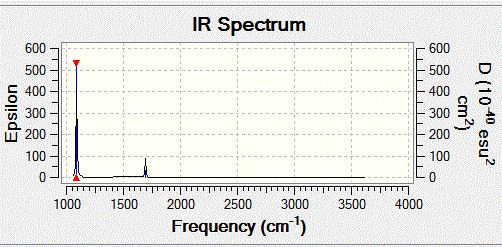
IR spectrum
There are two peaks in the IR spectrum, this explains there are two different chemical environments in ammonia molecules. One is on the N atom, the other indicates the three H atoms are in the same chemical enviornment
H2 information
Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -1.17853935 a.u. RMS Gradient Norm = 0.00003809 a.u. Point Group = D*H
Item Value Threshold Converged?
Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES Predicted change in Energy=-5.726834D-09
N2 information
Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -109.52359111 a.u. RMS Gradient Norm = 0.02473091 a.u. Point Group = D*H
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401007D-13
Reactivity
E(NH3)= -56.55776873 a.u. 2*E(NH3)= -113.11553746 a.u. E(N2)= -109.52359111 a.u. E(H2)= -1.17853935 a.u. 3*E(H2)= -3.53561805 a.u. ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.0563283 a.u. = -147.89 kJ/mol The reactivity energy of NH3 is -147.89 kJ/mol, which indicates the formation of NH3 is an exothermic reaction
PH5
Optimisation information
Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -344.23164586 a.u. RMS Gradient Norm = 0.02651592 a.u. Point Group = D3H
Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000055 0.001800 YES RMS Displacement 0.000022 0.001200 YES Predicted change in Energy=-1.032823D-09
Structure
test molecule |
Bond length = 1.43316/1.48687 a.u. Bond angle = 120.00/90.00000
Charge on atoms
charge on the P-atom = 0.412 charge on the H-atom = -0.183/-0.015
IR spectrum
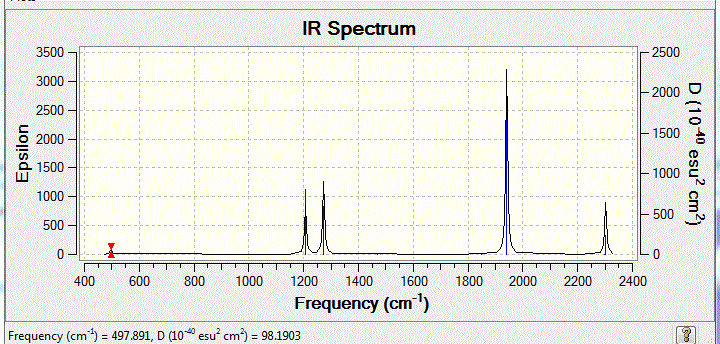
H2 information
Calculation Method = RB3LYP Basis Set = 6-31G(d,p) E(RB3LYP) = -1.17853935 a.u. RMS Gradient Norm = 0.00003809 a.u. Point Group = D*H
Item Value Threshold Converged?
Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES Predicted change in Energy=-5.726834D-09
P information
Calculation Type = SP Calculation Method = UB3LYP Basis Set = 6-31G(d,p) Spin = Doublet E(UB3LYP) = -341.19416476 a.u. RMS Gradient Norm = 0.00000000 a.u. Point Group = OH
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy= 0.000000D+00