William
NH3
Optimisation of Molecular Model
Calculation Method:RB3LYP
Basis Set:6-31G(d,p)
E(RB3LYP):-56.55640542 a.u.
RMS Gradient Norm:0.00000485 a.u.
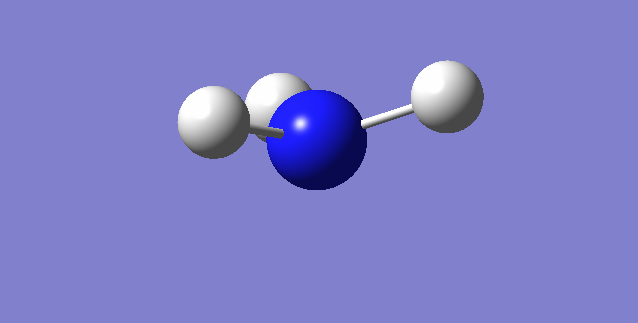
Point Group:C3V
Bond length: 1.10798 a
Bond Angle: 105.74116 o
Link:File:WDMNAISMITH NH3 OPTF POP.LOG
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986278D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.018 -DE/DX = 0.0 !
! R2 R(1,3) 1.018 -DE/DX = 0.0 !
! R3 R(1,4) 1.018 -DE/DX = 0.0 !
! A1 A(2,1,3) 105.7412 -DE/DX = 0.0 !
! A2 A(2,1,4) 105.7412 -DE/DX = 0.0 !
! A3 A(3,1,4) 105.7412 -DE/DX = 0.0 !
! D1 D(2,1,4,3) -111.8571 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Table 1. Item of NH3
Figure 1. Molecular model of Ammonia |
Vibrations
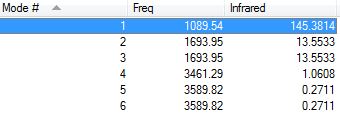
Table 2. Vibrational modes of ammonia
From the 3N-6 rule, 6 vibrations would be expected (3(4)-6). There are four stretching modes (4,5,6) and two bending modes (1,2,3). Bending modes 2 and 3 are degenerate as are the stretching modes 5 and 6 as they vibrate at the same frequency. Mode 4 is highly symmetric and mode 1 is known as the "umbrella mode". All vibrations are accompanied by a change in dipole moment, however, the intensity of modes 4,5,6 is too low to be observed and modes 2 and 3 vibrate at the same wave number. Therefore, only 2 peaks would be expected experimental spectrum of gaseous ammonia.
In the experimental IR spectrum of ammonia, absorptions at 1630 cm-1. and a doublet at 932 and 965 cm-1 are observed.[1] The doublet is caused by the "umbrella" vibrational mode. The nitrogen atom can tunnel through the centre to invert the molecule as shown below.[2] The two different states give rise to a doublet.
Figure 2. The extremes of the "umbrella" vibration mode of ammonia.
Atomic Charges
The charge of the N atom -1.125 and the H atoms is 0.375. As N is more electronegative element, it is expected to have a greater negative charge than H as it attracts more electron density. Moreover, the sum of the atomic charges is 0, as it is neutral molecule.
Haber-Bosch Process
N2
Calculation Method:RB3LYP
Basis Set:6-31G(d,p)
E(RB3LYP):-109.52412868 a.u.
RMS Gradient Norm:0.00000060 a.u.
Point Group:D∞H
Nitrogen gas has a stretching mode at at 2457.33 cm-1.
Link:File:WDMN N2 OPTF POP.LOG
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401010D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1055 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Table 3. Item of N2
H2
Calculation Method:RB3LYP
Basis Set:6-31G(d,p)
E(RB3LYP):-1.17853936 a.u.
RMS Gradient Norm:0.00000017 a.u.
Point Group:D∞H
Hydrogen gas has a stretching mode at at 4465.68 cm-1.
Link:File:H2 wdmn.LOG
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Table 4. Item of H2
Energy of Reaction
N2 + 3H2 -> 2NH3
E(NH3)=-56.55640542 a.u.
2*E(NH3)=-113.1128108 a.u.
E(N2)=-109.52412868 a.u.
E(H2)=-1.17853936 a.u.
3*E(H2)=-3.53561808 a.u.
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-139.3197527 kJmol-1
As the energy change is negative, the formation of ammonia is more favorable than the backwards reaction.
BH3
Optimisation of Molecular Model
Calculation Method:RB3LYP
Basis Set:6-31G(d,p)
E(RB3LYP:-26.61532364 a.u.
RMS Gradient Norm:0.00000211 a.u.
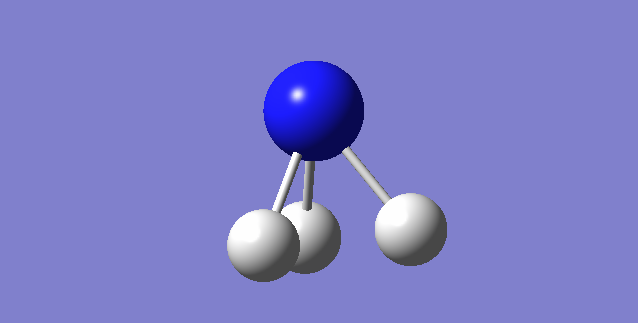
Point Group:D3H
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000017 0.001800 YES
RMS Displacement 0.000011 0.001200 YES
Predicted change in Energy=-1.053676D-10
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.1923 -DE/DX = 0.0 !
! R2 R(1,3) 1.1923 -DE/DX = 0.0 !
! R3 R(1,4) 1.1923 -DE/DX = 0.0 !
! A1 A(2,1,3) 120.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 120.0 -DE/DX = 0.0 !
! A3 A(3,1,4) 120.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 180.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
Table 5. Item of BH3
Figure 3. Molecular model of borane |
Vibrations
Table 6. Vibrational Modes of Borane
From the 3N-6 rule, 6 vibrations would be expected (3(4)-6). There are three stretching modes (4,5,6) and two bending modes (1,2,3). Bending modes 2 and 3 are degenerate as are the stretching modes 5 and 6 as they vibrate at the same frequency. Mode 4 is not accompanied by a change in dipole moment.
Atomic Charge
The charge of the B atom 0.297 and the H atoms is -0.099. As H is the more electronegative element, it is expected to have a greater negative charge than B as it attracts more electron density. Moreover, the sum of the atomic charges is 0, as it is neutral molecule.
Molecular Orbitals
Figure 4. MO1
This MO is formed from the 1s AO of B. It is too low in energy too interact with the 1s AO of H and so is not involved in bonding.
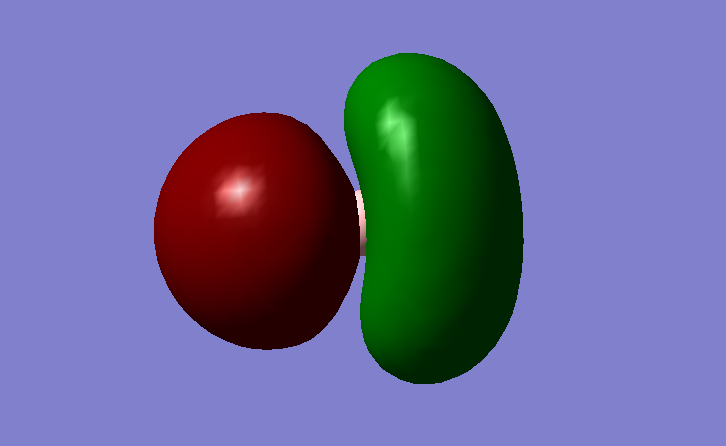
Figure 5. MO2
The MO is formed by the combination of B 2s AO and the 1s AOs of the H atoms. The shape of the MO is heavily dependent on that of the H 1s AO as its valence AOs are lower in energy because H is more electronegative than B.
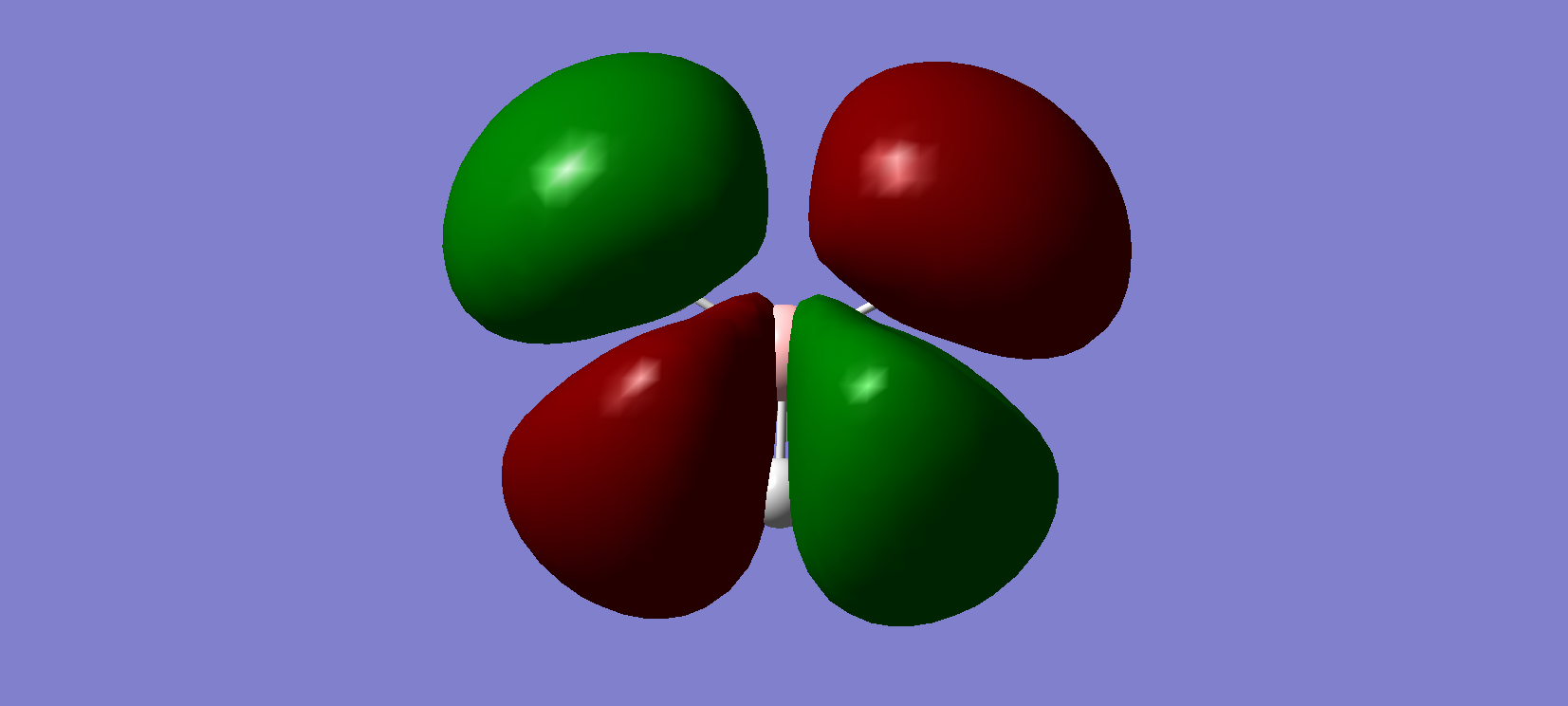
Figure 6. MO3 and MO4
These degenerate MOs are the combination of in phase overlap two of B 2p AOs and the H 1s AOs. They are also the HOMO.
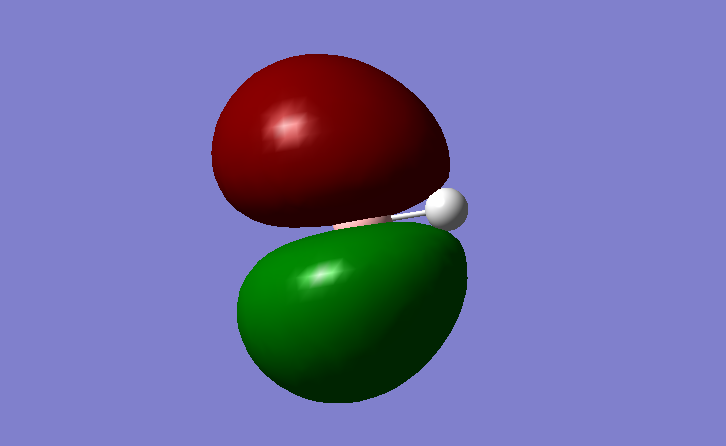
Figure 7. MO5
The non-bonding B 2p AO is the LUMO. As the LUMO is a non-bonding rather than an anti-bonding MO, it is lower in energy meaning that it can more easily accept electrons (Lewis base) from a HOMO without affecting the bonded hydrogen.
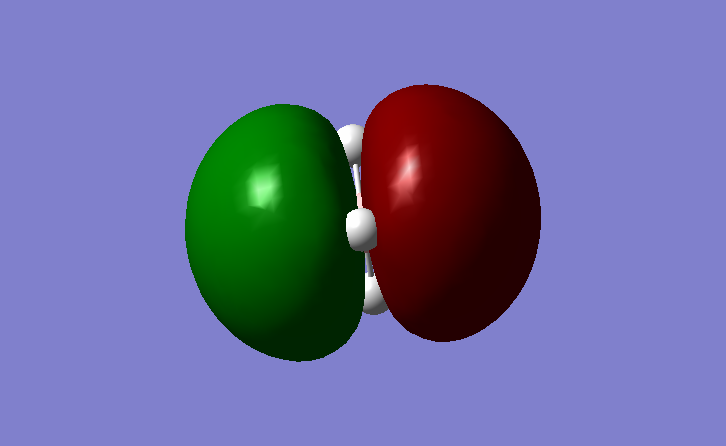
Figure 8. MO6
This MO is the anti-bonding orbital. It is much higher in energy than the bonding MOs.
O2
Calculation Method:RB3LYP
Basis Set:6-31G(d,p)
E(RB3LYP):-150.25742434 a.u.
RMS Gradient Norm:0.00007502 a.u.
Point Group:D∞H
Oxygen gas has a stretching mode at at 1642.74 cm-1.
Link:File:O2 WDMN OPT.LOG
Item Value Threshold Converged?
Maximum Force 0.000130 0.000450 YES
RMS Force 0.000130 0.000300 YES
Maximum Displacement 0.000080 0.001800 YES
RMS Displacement 0.000113 0.001200 YES
Predicted change in Energy=-1.033738D-08
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.216 -DE/DX = -0.0001 !
--------------------------------------------------------------------------------
Table 6. Item of O2
Figure 9. Molecular model of oxygen |
References
- ↑ 1.0 1.1 http://webbook.nist.gov/cgi/cbook.cgi?ID=C13283313&Mask=800. Date accessed 19/02/2016.
- ↑ 2.0 2.1 http://mysite.du.edu/~jcalvert/phys/ammonia.htm. Date accessed 19/02/2016.