Vince's page
VHP: introduction to molecular modelling
Introduction
In this workshop I analysed the molecules NH3, N2, H2, ClF, and COFCl (a product of the reaction of ClF with CO) using Gaussian. The process involves drawing and optimizing the molecule, and then investigating vibrations, charge distribution, and for ClF the Molecular orbitals of the compounds.
NH3 Molecule
Optimization information
| Name | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -56.55776873 (a.u) |
| RMS Gradient Norm | 0.00000485 (a.u) |
| Point group | C3V |
| Bond Length | 1.01798 (Angstroms) |
| Bond angle (H-N-H) | 105.741 (degrees) |
Convergence table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 molecule:
test molecule |
link to file: File:VHP NH3 OPT POP.LOG
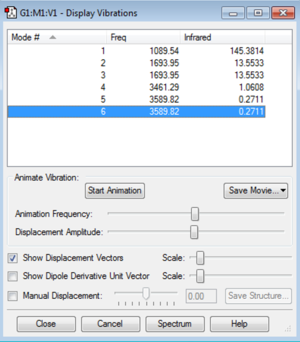
Vibrational information
Display vibrations:
- According to 3N-6 rule, there are 6 vibrational modes
- Modes 2-3 and 5-6 are degenerate
- Modes 1/2/3 are bending vibrations
- Modes 4/5/6 are bond stretch
- Mode 4 is highly symmetric with almost no change in dipole
- Mode 1 is the umbrella mode
I would expect to see 2 bands in an experimental spectrum: due to 3N-6 rule and 2 pairs of vibrations being degenerate (remembering that degenerate nodes give the same frequency),there are 4 unique frequencies given by the 6 vibrational nodes. The intensity of modes 1 and 2/3 are ~145 and ~26 while the peaks given by 4 and 5/6 are ~1 and ~0.5 respectively, this very large difference means the peaks given by 4 and 5/6 won't be visible on the spectrum containing the much larger peaks from modes 1 and 2/3. The reason for this difference is that modes 1 2 and 3 give rise to a large change in dipole, while modes 4/5/6 cause very little change in dipole.
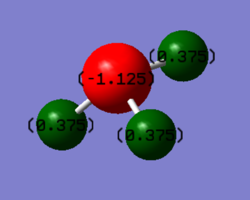
Charge distribution
Charge distribution showed that the Hydrogens had +0.375 each, and the Nitrogen had -1.125 charge.
I would expect nitrogen, being significantly more electronegative than hydrogen, to have a negative charge, and the hydrogens to have positive charge, the total charge should of course equal zero since NH3 is a neutral molecule.
N2 molecule
Optimization information
| Name | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -109.52412868 (a.u) |
| RMS Gradient Norm | 0.00000158 (a.u) |
| Point group | D(inf)H |
| Bond Length | 1.10550 (Angstroms) |
Convergence table
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000001 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
N2 Molecule:
test molecule |
Link to file: File:VHP N2 OPT POP.LOG
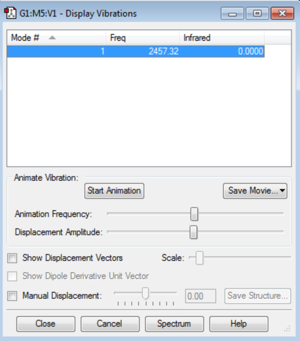
Vibrational information
display vibrations:
There is one vibrational mode that has an intensity of zero, since there is no dipole. I would expect there to be no peaks in the experimental spectrum due to the only mode giving zero intensity.
Charge distribution
N2 is a diatomic element, both atoms are identically electronegative so would have no difference in charge.
H2 Molecule
Optimization information
| Name | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -1.17853935 (a.u) |
| RMS Gradient Norm | 0.00003809 (a.u) |
| Point group | D(inf)H |
| Bond Length | 0.74289 (Angstroms) |
Convergence table
Item Value Threshold Converged? Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES
H2 Molecule:
test molecule |
Link to file: File:VHP H2 OPT POP.LOG
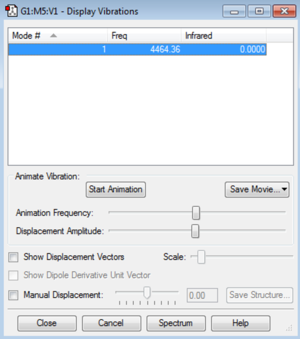
Vibrational Information
Display vibrations:
There is one vibrational mode that has an intensity of zero, since there is no dipole. I would expect there to be no peaks in the experimental spectrum due to the only mode giving zero intensity.
Charge distribution
H2 is another diatomic element, both atoms are of identical electronegativity so have identical charge.
Haber process
For reaction N2 + 3H2 -> 2NH3
E(NH3) = -56.55776873 a.u
2*E(NH3) = -113.11553746 a.u
E(N2) = -109.52412868 a.u
E(H2) = -1.17853935 a.u
3*E(H2) = -3.53561805 a.u
ΔE=2*E(NH3)-[E(N2)+3*E(H2)] = -0.05579073 a.u = -146.478561615 kJ/mol
The NH3 product is more stable than the reactants, the enthalpy of formation is negative showing that the products are in a lower energy state than the reactants.
ClF molecule
Optimization information
| Name | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -559.94269587 (a.u) |
| RMS Gradient Norm | 0.00005329 (a.u) |
| Point group | C(inf)V |
| Bond Length | 1.66371 (Angstroms) |
Convergence table
Item Value Threshold Converged? Maximum Force 0.000092 0.000450 YES RMS Force 0.000092 0.000300 YES Maximum Displacement 0.000162 0.001800 YES RMS Displacement 0.000229 0.001200 YES
ClF molecule:
test molecule |
Link to file: File:VHP CLF OPT POP.LOG
Vibrational Information
Display Vibrations:
There is only one mode because this is a diatomic linear molecule, the mode is a stretching mode. The dipole change is small because the electronegativity difference is relatively small.
Charge distribution
Fluorine (3.98 on the pauling scale) is more electronegative than Chlorine (3.16 on pauling scale), therefore F will pull bonding electron density towards itself, giving it a negative charge, which should be equal and opposite to Cl as the molecule is neutral. This is supported by the values calculated by the Gaussian.
Molecular orbitals
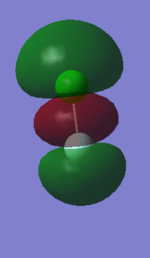
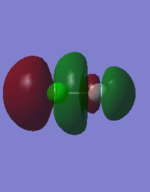
1:
This orbital is the bonding interaction of the two 3s orbitals from the atoms, it is quite deep in energy relative to some of the other filled orbitals.
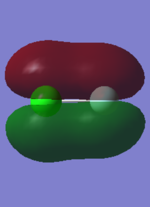
2:
This is a filled sigma bonding orbital, the orbital is not very deep in energy. The electron density between the nuclei contribute to holding the molecule together. It is a result of the interaction of the 3pz orbitals from the two atoms.
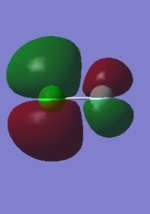
3:
This orbital is a filled bonding pi orbital generated by the 3py atomic orbitals. It is high in energy but not in LUMO/HOMO range. The 3px pi bonding orbitals are the same energy as this (they are degenerate). Since the antibonding orbitals for these are also filled they cancel each other out and overall don't contribute to the bonding.
4:
This is an antibonding pi ortibal, generated by 3py AO's, this and the degenerate 3px anti pi form the HOMO. This is the orbital that is cancelling out the 3py pi bonding orbital above.
5:
This is the LUMO of the molecule, it is the antibonding part of the sigma orbital (number 2 above), if this was filled it would take away from the bonding by cancelling out the sigma interaction, but it does nothing as the molecule is since it is unfilled.
Reaction with CO
ClF is a versatile fluorinating agent used to convert metals and non-metals to their fluorides. Another note is the reaction of ClF with CO produces Carbonyl chloride fluoride. [1] I created and analysed this molecule in Gaussian:
Optimization information
| Name | Value |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy | -673.36045263 (a.u) |
| RMS Gradient Norm | 0.00025602 (a.u) |
Carbonyl chloride fluoride:
test molecule |
Link to file: File:VHP EXTRA MOLECULE POP.LOG
Convergence table
Item Value Threshold Converged? Maximum Force 0.000417 0.000450 YES RMS Force 0.000244 0.000300 YES Maximum Displacement 0.001407 0.001800 YES RMS Displacement 0.000735 0.001200 YES
Display vibrations
As predicted by the 3N-6 rule there are 6 vibrational modes. None of these are degenerate, but I would still expect only 4 peaks on the experimental spectrum, as modes 1 and 2 only give a tiny change in dipole, compared to the peak from 5 they would be invisible.
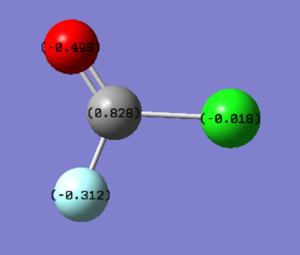
Charge distribution
Interestingly the fluorine atom doesn't have the most negative charge, despite being more electronegative than oxygen, this is because the oxygen is closer to the carbon atom, being double bonded to it, so can withdraw more of the electron density. Also there is a filled pi orbital for the oxygen to draw more electron density away from the oxygen. These outweigh the small difference in electronegativity between oxygen and fluorine, meaning oxygen has relatively more electron density around it than fluorine.