User:Zc2814
'Computational Experiment of Transition States''''
Introduction
Not all the times can we observe the true transition state of a reaction. Computing a reaction help us to visualize a transition state of the very reaction we are looking at, and can even predict the favoured routine of it by calculating the energy levels. This experiment includes three Diels-Alder type reactions, by computing different TSs, this might reveal the pathway of a true reaction.
Nf710 (talk) 09:11, 21 November 2016 (UTC) You should have talked about How to determine a transition state here, and talking about the methods would have bee good.
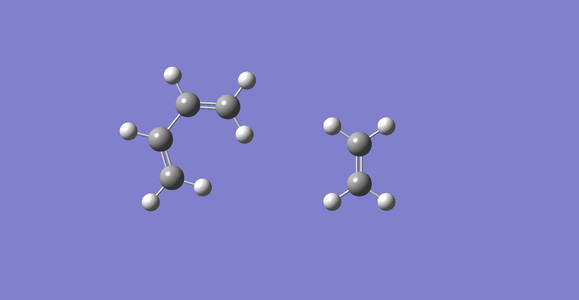
Exercise 1:Reaction of butadiene with ethene
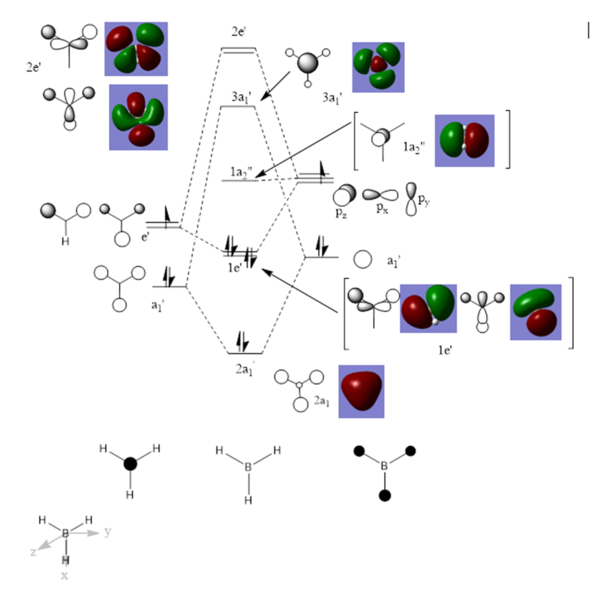 Diagram of expected mixing of certain MOs and energies during the reaction.
Diagram of expected mixing of certain MOs and energies during the reaction.
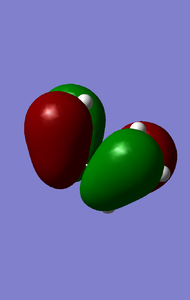
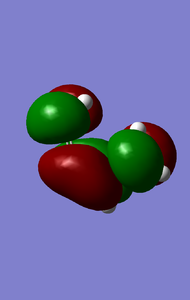 MOs of reactants that involved in the reaction(-0.351,0.01105kJ/mol).
MOs of reactants that involved in the reaction(-0.351,0.01105kJ/mol).
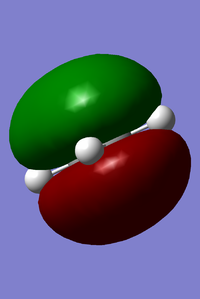
 MOs of reactants that involved in the reaction(-0.39220,0.04256kJ/mol).
MOs of reactants that involved in the reaction(-0.39220,0.04256kJ/mol).
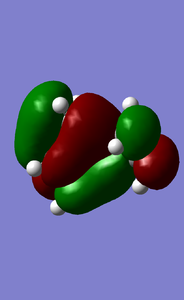

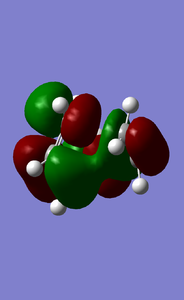
 MOs of the reaction transition state(-0.32755,-0.32534,0.01731,0.03067kJ/mol).
MOs of the reaction transition state(-0.32755,-0.32534,0.01731,0.03067kJ/mol).
Above shows the expected interaction of MOs and computed ones. The shown expected and computed MOs are of different energy levels and relationship, indicating different MOs interacted. This is because the model given to the computer is not very close to reality and the computing method used in Gaussian has its limitation. However, this does not affect the general rule of "g-g" "u-u" interation. "g-u"does not interact, or, the interaction gives a wave function equals to 0. Thus, the requirement for reaction symmetry is to let the MO pair or pairs which shall interact, approaching each other at right orientation.
Bond Length Change during reaction(Angstrom)
| butadiene | ethene | TS butadiene | TS ethene | Tipical sp2 | Tipical sp3 | |
|---|---|---|---|---|---|---|
| C=C | 1.33523,1.33530 | 1.32731 | 1.35729,1.35732 | 1.45000 | 1.35520 | / |
| C-C | 1.46852 | / | 1.43938 | / | / | 1.54000 |
The data give idea of how the C-C bond lengths and their hybridization change through the reaction pathway. The C=C stretches and C-C shrinks comparing starting material and TS. Comparing these with "Tipical sp2 C-C" and "Tipical sp3 C-C", we can probably conclude that this is the same as changing in hybridization. C=C is sp2 hybridized and it becomes longer, indication a increasing in sp3, or p character. C-C is sp3 hybridized and it's decrease in length indicates the increase in sp2, or increase in s character. The final product shows that the 3 C=C ends in single bonds and the C-C becomes C=C. The Van der Waal radius for C is 170pm[1] and it is larger than TS carbon bonds. The Van der Waal radius comes from averaging a C-C single bond, however in the TS, the C bonds are bearing double to single or opposite transition. No wonder Van der Waal radius has a larger value.
Nf710 (talk) 09:20, 21 November 2016 (UTC) This section was done quite well, you could have used jmols to display the orbitals
Exercise2:Cyclopentadiene with Benzoquinone
| benzoquione | cyclopentadiene | exo TS | exo product | endo TS | endo product | |
|---|---|---|---|---|---|---|
| energy(kJ/mol) | 0.005536 | 0.111695 | 0.179487 | 0.107283 | 0.180125 | 0.108082 |
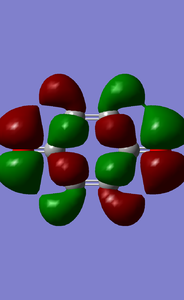
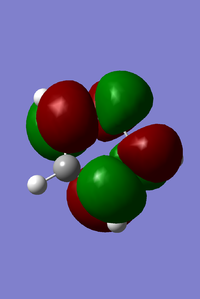
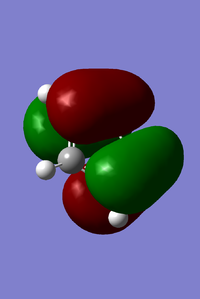
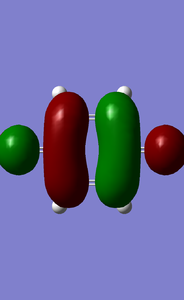 MOs of benzoquinone associated with D-A reaction
MOs of benzoquinone associated with D-A reaction
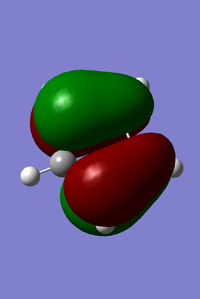
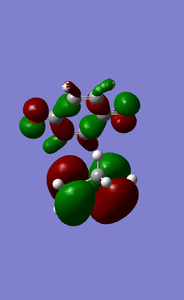
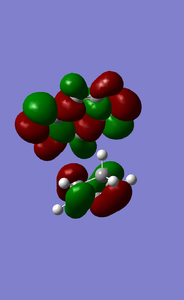
 MOs of cyclopentadiene associated with D-A reaction
MOs of cyclopentadiene associated with D-A reaction
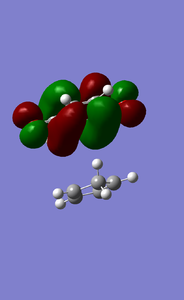
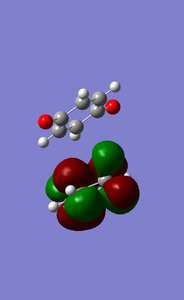 MOs of exo D-A reaction transition state
MOs of exo D-A reaction transition state
| reactants | exo reaction barrier | exo reaction energy | endo reaction barrier | endo reaction energy | kinetically favoured | thermodynamically favoured | |
|---|---|---|---|---|---|---|---|
| energy(kJ/mol) | 0.117231 | 0.062256 | -0.009948 | 0.062894 | -0.009149 | exo | exo |
09:31, 21 November 2016 (UTC) Yo should have used B3LYP here. PM^ is not a good enough method it is too parameterised. It should typically be used for finiding initial geometries for higher level calculations. or calculations on large molecules.
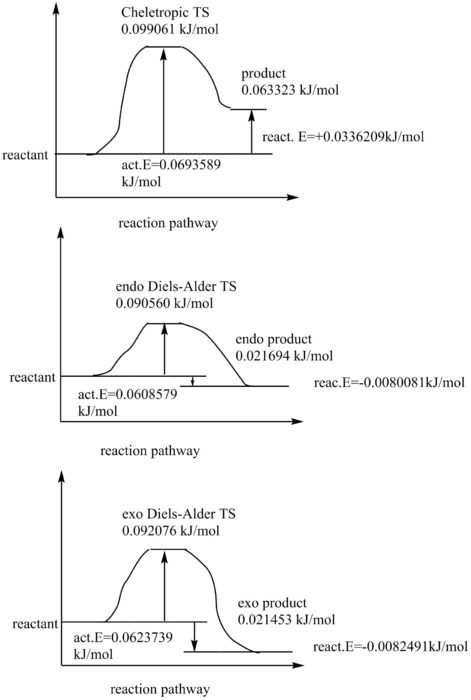
Exercise3:O-Xylylene with Sulphur Dioxide
(You can't simply write "kJ/mol" after an energy. The units are in Hartree and the conversion is ~2625kJ/mol to the Hartree Tam10 (talk) 13:55, 11 November 2016 (UTC))
(Something has gone wrong with your cheletropic calculations. You shouldn't be getting a positive reaction energy. It looks like the pathway is reversed Tam10 (talk) 13:55, 11 November 2016 (UTC))
Cheletropic reaction is strongly disadvantaged comparing to Diels-Alder reactions, due to high activation energy and positive reaction energy which gives its endothermic nature. With the two Diels-Alder reactions, there is a small difference between exo and endo pathways. The endo has a slightly lower activation energy due to the stabilization at the back of dienen part during TS, so endo is the kinetically favored product. However, endo is not the thermodynamically favored one, as the exo one has a lower, or more negative reaction energy. Both endo and exo have negative reaction energy and are exothermic, but exo gives out more energy therefore more stable. During the reaction, the diene part in the 6-membered ring together with the new-formed C=C in the very ring forms resonance structure. In the computed TS we can see that the whole ring is surrounded by dotted line. In a resonance structure, all bonds are of the same length, between typical C-C and C=C. The carbon bond lengths vary during the reaction as the C-Cs and C=Cs need to end in a same length. This is shown in the GIF of IRC pathway. However, if we cyclize at the diene part in the ring, the will not be any benefit from resonance, thus this is chosen against.
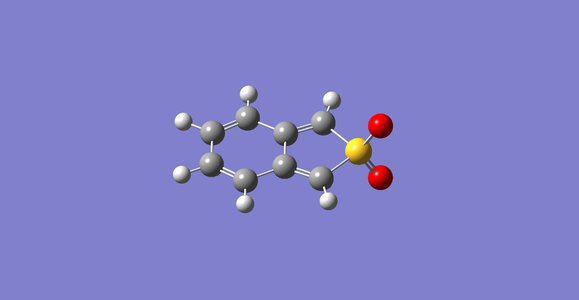 semi-empirical optimized cheletropic product.
semi-empirical optimized cheletropic product.
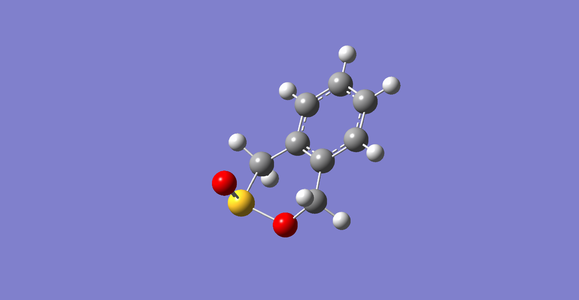 semi-empirical optimized endo D-A product
semi-empirical optimized endo D-A product
 semi-empirical optimized exo D-A product
semi-empirical optimized exo D-A product
 IRC pathway of cheletropic reaction
IRC pathway of cheletropic reaction
Conclusion
The computation is a powerful method. It indeed can help in visualizing, calculating energies, structures and predictions. However, it is also very important to realize that this method has its limitation, in ways of calculation, in the model inbeded, in the limited information you gave. This experiment also looked at how molecular orbitals join and how bond length change during the very three reactions.The MOs have to be both 'g' or both 'u', of close energies, and at right orientation to mix together.