User:Xv114
Introduction
A transition state is a transient species with the highest energy in the reaction profile that cannot be isolated or detected by spectroscopic methods. As a result it is the maximum point in the potential energy surface with zero gradient. This wiki page would discuss about transition states of cycloaddition reaction between different reactants using Gaussview for transition state optimisation followed by IRC and frequency calculation to determine the energy of reactants, transition states and products to determine favourable reaction route.
Nf710 (talk) 16:43, 20 January 2017 (UTC) This is not enough detail you need to talk about second derivatives etc.
Exercise 1: Reaction between butadiene and ethylene
Reaction Scheme
Butadiene and ethylene react in Diels Alder as shown in Scheme 1:

Molecular orbital
The highest occupied molecular orbitals (HOMO) and lowest occupied molecular orbitals (LUMO) of both butadiene and ethylene shown in Figure 1, can overlap to a give 4 different transition state MOs, which correlates with those illustrated on the MO diagram, Figure 2.


From the MO diagram, it is the LUMO of butadiene that interacts with HOMO of ethylene.
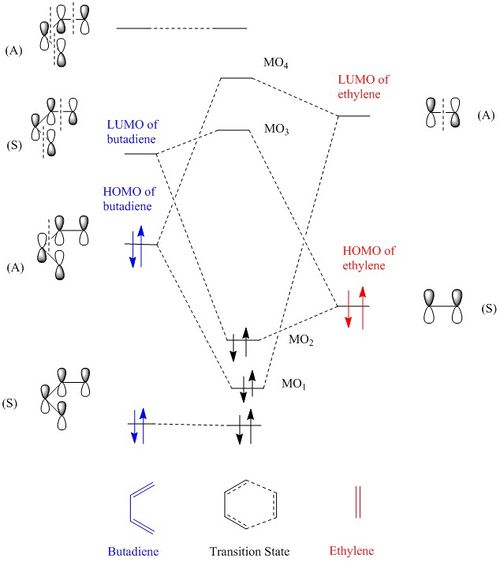
From Figure 2 and 3, it can be concluded that symmetry of MO of both reactants needs to be the same in order for overlap to occur and in turn the reaction would be 'allowed'. I.e. Only symmetric orbital can overlap with another symmetric orbital, not with asymmetric. This explains why only LUMO of butadiene and HOMO of ethylene can overlap with each other due to both having the same symmetry.Thus, the orbital overlap integral for a symmetric-asymmetric interaction would be zero, whereas that for symmetric-symmetric or asymmetric-asymmetric interaction would be non-zero.[1]
Bond Length
Changes in bond length can be monitored to determine whether reactants have undergone a reaction.
| Typical carbon-carbon bond length : | Å |
|---|---|
| Sp3-sp3 | 1.54 |
| Sp3-sp2 | 1.50 |
| Sp2-sp2 | 1.34 |
Van der waal radius for carbon: 1.70 Å [3]
| Bond length (Å) | |||
|---|---|---|---|
| Bond number | Reactant | Transition state | Product |
| 1 | 1.32 | 1.38 | 1.54 |
| 2 | 0 | 2.11 | 1.54 |
| 3 | 1.33 | 1.38 | 1.50 |
| 4 | 1.47 | 1.41 | 1.33 |
| 5 | 1.33 | 1.38 | 1.50 |
| 6 | 0 | 2.11 | 1.54 |
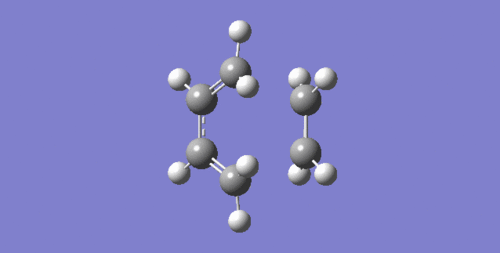
The bond lengths in both reactants and product in Figure 4 correlate with those in Table 2. However the C=C bonds lengthen in transition state to a length that is between a typical sp3-sp2 and sp2-sp2 C-C bond length before finally becoming single C-C bond in product.
Transition state vibrations
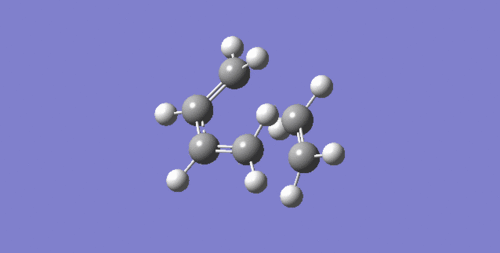
According to Figure 5, the vibration of transition state at -949 Hz indicate that the formation of two bonds appear to be synchronous with both carbons of diene and dienophile approaching each other simultaneously. In contrast to vibration at 145 Hz the formation of bond is asynchronous.
IRC

Nf710 (talk) 17:08, 20 January 2017 (UTC) Key points are there, minimal explanation.
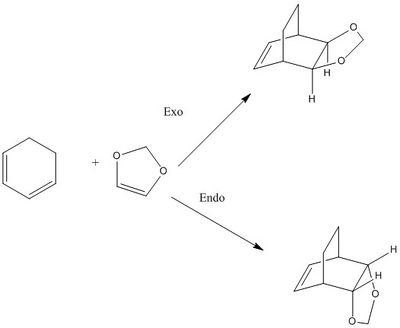
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Reaction Scheme
Cyclohexadiene and 1,3-dioxole can react with each other in Diels Alder reaction as shown in Scheme 2:
Molecular orbitals
The MO diagram for reaction between cyclohexadiene and 1,3-dioxole in Figure 8 below:
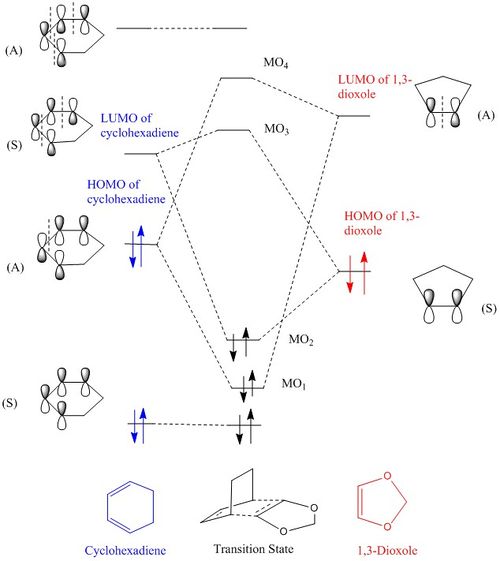
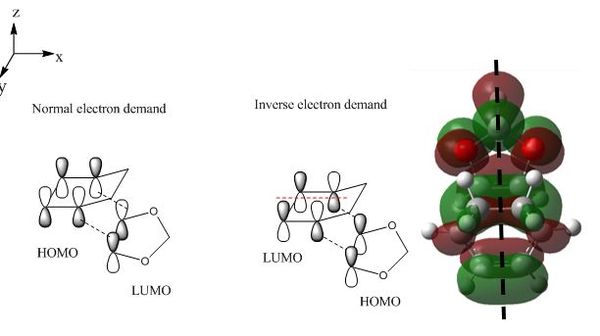
The three dimensional transition state orbitals visualised in Gaussview in Figure 10-13 correlate with those in the MO diagram, Figure 8. The presence of oxygen lone pair would raise the energy levels of dioxole, including its HOMO. As a result energetically the HOMO of dioxole would interact better with LUMO of cyclohexadiene.[4] Furthermore presence of mirror plane extending along the length of the transition state is consistent with that for a cycloaddition reaction with an inverse electron demand as illustrated in Figure 14 confirming that the reaction has an inverse electron demand.
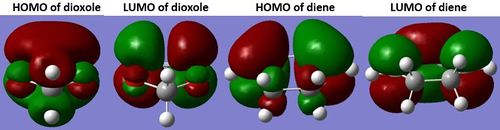
(You're using the PM6 orbitals here. They are quite different to B3LYP Tam10 (talk) 14:14, 4 January 2017 (UTC))

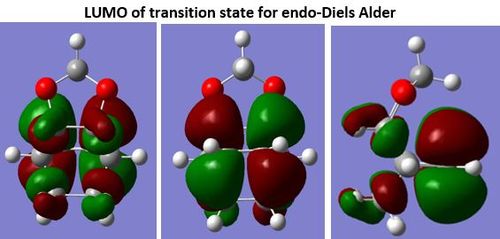
(This is the endo product Tam10 (talk) 14:14, 4 January 2017 (UTC))
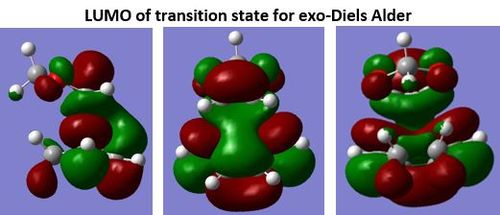
| Reaction | Activation energy (kJ/mol) | Gibbs free energy (kJ/mol) |
|---|---|---|
| Endo-Diels Alder | 151 | -76.2 |
| Exo-Diels Alder | 159 | -72.3 |
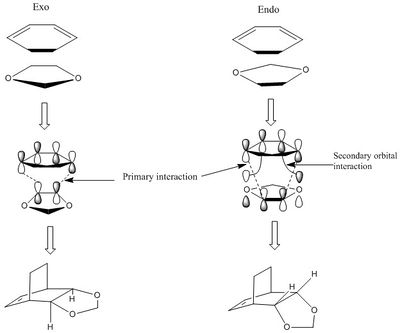
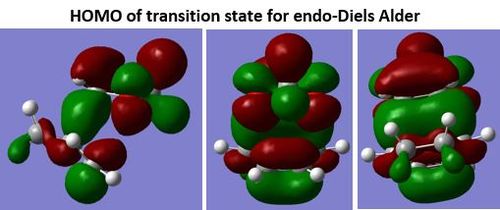
Secondary orbital interaction is observed in the circled region between oxygen lone pair and π* orbital as shown in Figure 16. The presence of secondary and primary orbital interactions, as shown in Figure 16 and 15, and from the lower activation energy and Gibbs free energy calculated in Table 2 means the endo-Diels Alder reaction is both the thermodynamic and kinetic product. This also suggests that there is a lower steric interaction in endo- than exo-Diels Alder reaction.

Nf710 (talk) 17:57, 20 January 2017 (UTC) Good second section your energies are slightly out but you have got the correct conclusion, good description of SSO
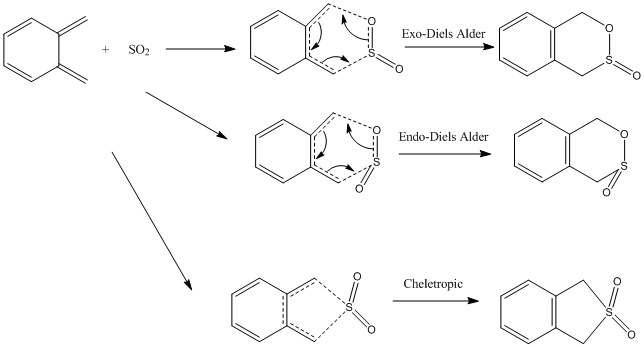
Exercise 3: Diels Alder vs Cheletropic
Reaction Scheme
Xylene and sulphur dioxide can undergo either Diels-Alder or cheletropic reaction, forming six membered ring and a five membered ring respectively, as shown in the scheme below.
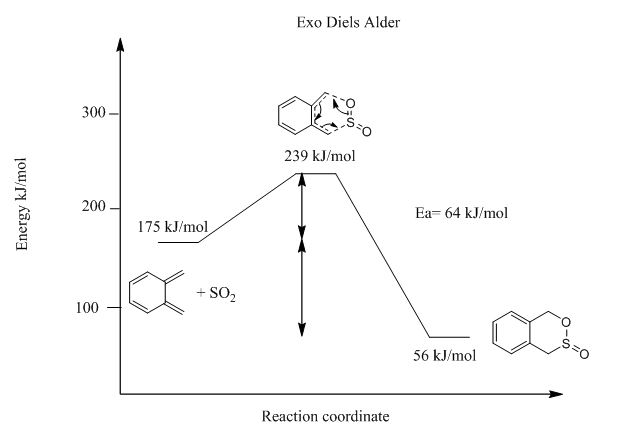
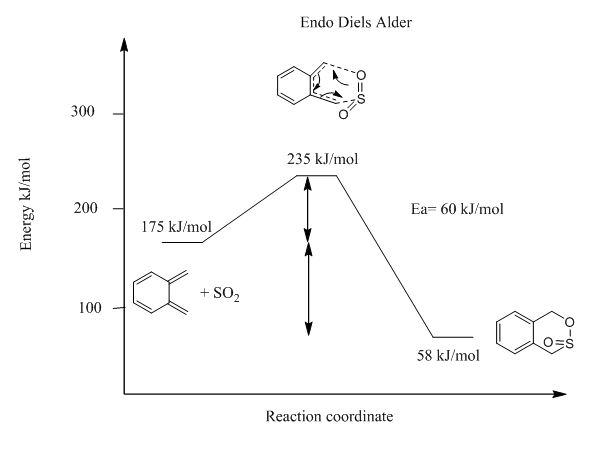
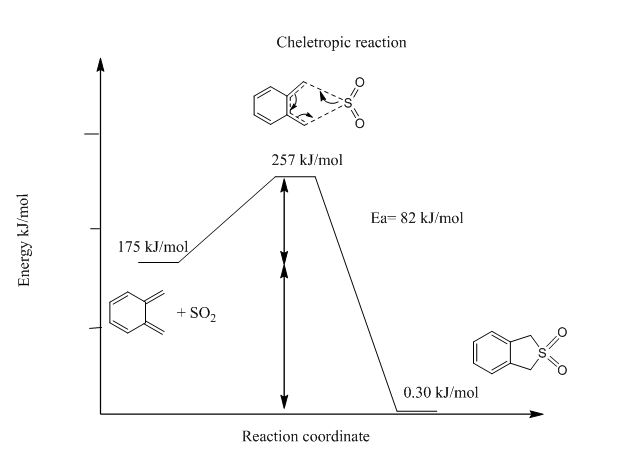
From Table 3 and Figure 17-19, it can be deduced that endo-Diels Alder reaction has an activation barrier that is lower than that of exo-Diels Alder reaction by 4 kJ/mol, however the endo-Diels Alder product is higher in energy than its exo counterpart. Cheletropic product on the other hand is has the greatest activation barrier as well as Gibbs free energy. This suggests that kinetically endo-Diels Alder is the preferred reaction route but cheletropic reaction produces the most thermodynamically stable product.
| Reaction type | Activation barrier (kJ/mol) | Gibbs Free energy (kJ/mol) |
|---|---|---|
| Endo- Diels Alder | 60 | -117 |
| Exo-Diels Alder | 64 | -120 |
| Cheletropic | 82 | -176 |
From Figure ? below, it is also evident between the two types of Diels-Alder reactions, the endo-Diels Alder product is the kinetic product and exo product is the thermodynamic product.
(Best to put these on the same coordinate and normalising to the reactants ie setting them to 0 Tam10 (talk) 14:14, 4 January 2017 (UTC))
IRC
The IRC calculations below were obtained after PM6 optimisation of the transition states for each reaction shown in Scheme 3.

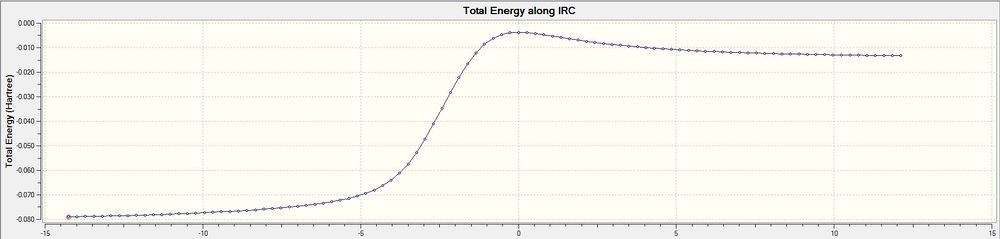

For all three IRC calculations shown above in Figure 21-22, the energy of IRC is highest when the first partial double bond is formed in the cyclohexadiene ring before the the formation of C-S and C-O sigma bonds, this is then followed by formation of partial bond in the exocyclic double bond and SO2, and full aromaticity is established at the end of the reaction to produce a benzene ring. Figure 21 shown is different from Figure 20 and 22 by starting off with a higher energy, because IRC calculation begin with the exo product and ends with the breaking of 2 sigma bonds to give the reactants.
Reference
- ↑ J.Clayden, N.Greeves, S. Warren, Organic Chemistry, OUP, New York, 2012, 2nd. ed,
- ↑ Fox, Marye Anne; Whitesell, James K. (1995). Organische Chemie: Grundlagen, Mechanismen, Bioorganische Anwendungen. Springer. ISBN 978-3-86025-249-9.
- ↑ A. Bondi. "Van der Waals Volumes and Radii". J. Phys. Chem. ,1964, 68 (3): 441–51.
- ↑ J.Clayden, N.Greeves, S. Warren, Organic Chemistry, OUP, New York, 2012, 2nd. ed, pg. 887
