User:Tc1812
Introduction
Computational chemistry has been an important sub-discipline within chemistry. It has been frequently used to support the theory and results. Based on the computational program and theoretical calculation, the major application of computational chemistry is to predict the thermodynamic motion of reaction , stereo-chemistry of compounds , molecular orbital calculation and transition structure. Transition structure, also called as activated complex, is a significant conformation on the process of reaction. Obviously,potential energy of the transition state is the highest (Is it really the highest? What happens if two atoms completely overlap in space? Does that mean it is a transition state? João (talk) 18:17, 14 April 2015 (BST)), illustrated by energy surface diagram or reaction profiles. Furthermore, transition structure/state and the energy barrier are dependent on reactivity ,regioselectivity,and mechanism (Does it depend on, or does is determine these properties of the reaction? João (talk) 18:17, 14 April 2015 (BST)). This characteristic could be well performed in term of the Cope rearrangement and the Diels Alder reaction. One measure of the progress is to deter the length of bond being made or broken .However ,this method is no longer suitable for complicated compound .Therefore ,molecular orbital-based method will be adopted to estimate the transition structure.In this experiment,transition structure of Cope Rearrangement and Diels Alder reaction will be predicted by the Gaussian software respectively.1
Result and disscussion
Transition Structure of Cope Rearrangement
Optimization of Reactants and Products
The conformers of the 1,5-hexadiene was analysed by optimization at HF/3-21G level of theory. Point groups, electronic energy and relative energy(Note:1 Hartree = 627.509 Kcal/mol) corresponding to each conformer are illustrated in Table 1. Anti-periplanar structure is often regarded as the conformer with the lowest energy due to minimization of steric interaction . However , conformer gauche 3 possesses the lowest electronic energy among the conformers , resulted from the interaction of п orbital with vinyl protons. Anti 2 conformer has center of symmetry , as illustrated by point group Ci. It was then optimized with B3LYP/6-31G*. After optimization via B3LYP/6-31G*, the final structure was compared with structure from HF/3-21G . It is noticeable that the structure from optismation B3LYP/6-31G* possesses lower electronic energy , which is more stable (One cannot compare absolute energies calculated at different levels of theory. João (talk) 18:17, 14 April 2015 (BST)). It can explained by the structural geometry (The difference in energy is not due to the differences in the geometry but rather the difference in the method used. João (talk) 18:17, 14 April 2015 (BST)).The bond length does not give any support, even if the bond length slightly changed . The dihedral angle , also called as torsional angle, proves this fact.The dihedral angle (C1-C4)slightly increases , indicating carbon in position 1 and 6 away from each other. (Overall, are the differences in geometry big or small? João (talk) 18:17, 14 April 2015 (BST))
| Conformer | Structure | Point Group | Energy/Hartrees HF/3-21G | Relative energy/Kcal/Mol | ||
| Gauche | C2 | -231.68772 | 3.10 | |||
| Gauche2 | C2 | -231.69167 | 0.62 | |||
| Gauche3 | C1 | -231.69266 | 0.00 | |||
| Gauche4 | C2 | -231.69153 | 0.71 | |||
| Gauche5 | C2 | -231.68962 | 1.91 | |||
| Gauche6 | C1 | -231.68916 | 2.20 | |||
| Anti1 | C2 | -231.69260 | 0.04 | |||
| Anti2 | Ci | -231.69254 | 0.08 | |||
| Anti3 | C2h | -231.68907 | 2.25 | |||
| Anti4 | C1 | -231.69091 | 1.06 |
In end of this exercise, frequency of anti 2 conformer at 298.15 K was obtained by same method B3LYP/6-31G*. None of imaginary frequency are illustrated in vibration list, hence ,the energy of this conformer is minimized. The energy of structure at 0 K could also be estimated by typing temp = 0.01 on windows with same method B3LYP/6-31G* in order to correct the energy. According to the table 3., the sum of electronic and zero-point Energies is same at either 0 K or 298.15 K. All types of energy are sames at 0 K , because energy only consists of zero-point energy(residual energy) and electronic energy at this situation .
| Type of energy/Hartrees | 0 K | at 298.15 K |
| The sum of electronic and zero-point Energies | -234.469219 | -234.469219 |
| The sum of electronic and thermal Energies | -234.469219 | -234.461869 |
| The sum of electronic and thermal Enthapies | -234.469219 | -234.460925 |
| The sum of electronic and thermal Free Energies | -234.469219 | -234.500808 |
Transition Structures of ' 'Chair' ' and ' 'Boat' ' Conformation
Cope-rearrangement is one type of [3,3]sigmatropic rearrangement.The bond breaking and formation occur simultaneously. Currently, Cope-rearrangement proceed via two acceptable transition structures.One is a ' 'boat' ' and the other is ' 'chair' '. Both of transition structures are illustrated in Figure 2.
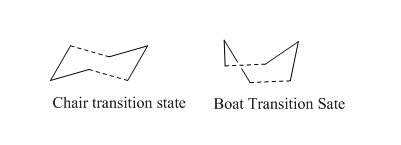
In this section, chair and boat transition structure were optimised by three different method .The method(a) is to calculate the force constant .The method (b) is about the application of redundant coordinate editor and the method(c) is QST2 .
' 'Chair' ' Transition Structure
An allyl fragment (CH2CHCH2) was optimised with HF/3-21G level of theory to generate a new transition structure.This new transition structure were copied twice and put into a new window.The intermolecular distance between two allyl fragments was adjusted to 2.2 Å. Therefore, ' 'Chair' ' transition structure(C2h) was successfully optimized at HF/3-21G level of theory and an imaginary frequency -817. 97 cm-1 was obtained. However, this method has a limitation. If the geometry of ' 'guess' ' transition structure is not really similar to the that of actual transition structure ,it will generate a undesired result or failure of programming. In same case, redundant coordinate editor will be used to generate a more acceptable transition structure by editing redundant coordinates (It is true that the frozen coordinate method is used to obtain a better guess for a transition state structure, but also a simpler calculation since we only calculate the force constants on the postulated reaction coordinate, instead of all molecular modes. João (talk) 18:17, 14 April 2015 (BST)).The chair structure was also run with the freezer-coordinate method,(shown in table 4.) In this method, the intermolecular distance was fixed at 2.2 Å , so the coordinates were freezed . The structure was optimised at HF/3-21G level of theory. Later on ,the the coordinates were unfreezed and the structure was optimised again.
| Structure | |
|---|---|
| Chair | 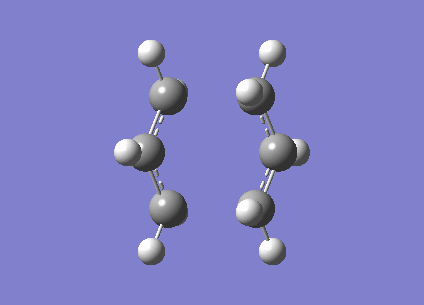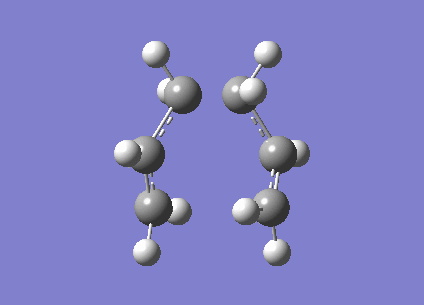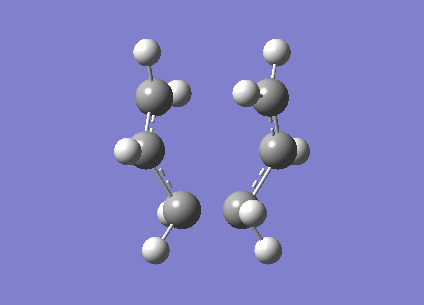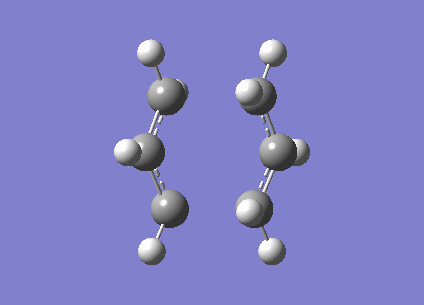
|
After running with freezer coordinate editor,the electronic energy of chair transition structure is -817.96 cm-1 (Is this an energy? João (talk) 18:17, 14 April 2015 (BST)),which is closer to the bond length from the method A (Is it a bond length now? João (talk) 18:17, 14 April 2015 (BST)). However, the bond length increases to 2.0270 Å from 2.02025 Å (Is this difference significant? João (talk) 18:17, 14 April 2015 (BST)). According to the literature, bond length between two allyl fragment is 2.086 Å (Reference? João (talk) 18:17, 14 April 2015 (BST)). Therefore, redundant coordinate editor can provide more accurate result.3.
' 'Boat' ' Transition Structure
In QST2 method, ' 'Boat' '(C2v) transition structure is found by interpolating between reactant and product . In this method, 1,5-hexadiene(antiperiplanar 2) is as the reactant and product, respectively. Atoms in reactant and product must be numbering. Furthermore, the geometry for product and reactant must be adjusted to be closer to the ' 'boat' ' transition structure by changing dihedral angle of C2-C3-C4-C5 to 100o,as shown below.
| Reactant | 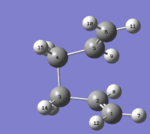 |
Product | 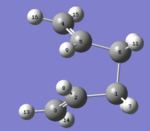 |
Boat transition structure | 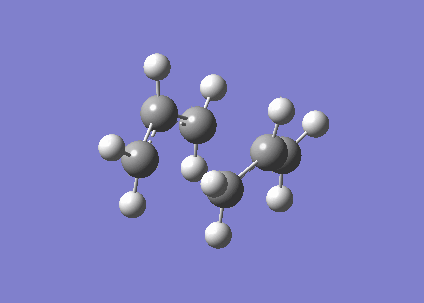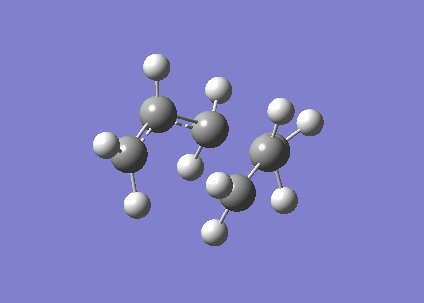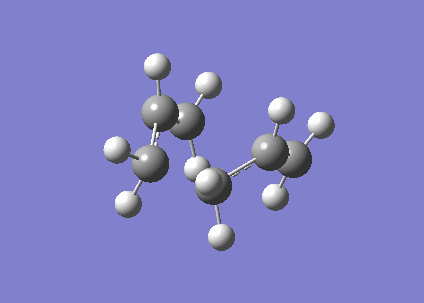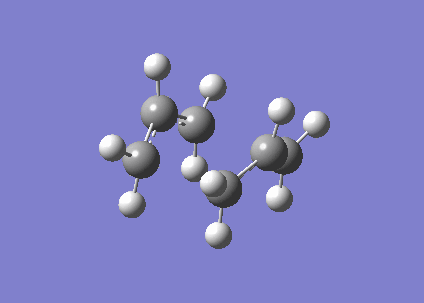
|
IRC(Intrinsic Reaction Coordinate)
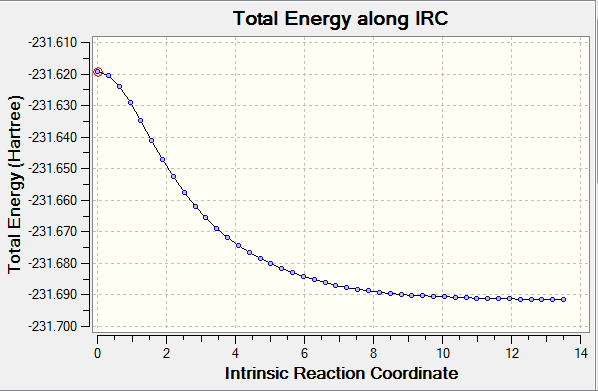
The IRC method allows to demonstrate the minimum pathway from a transition state to the reaction coordinate of the minimum energy. ' 'Chair' ' transition structure was run in the forward direction because reaction coordinate is symmetrical. Default increased to 50 from 6 , in order to illustrate the process in detailed. Finally, The force constant was adjusted to ' 'always' ' option.
 |
|
From the IRC, the conformer will undergo in gauche 2 or gauche 4 from the Appendix 1 (Which one is it? João (talk) 18:17, 14 April 2015 (BST)). Once the IRC finished, it did not reached a minimum geometry. The main function of IRC is to predict the trend where the slope of potential energy surface is steepest (This is indeed what the IRC calculation does, but why is this interesting from the chemical point of view? João (talk) 18:17, 14 April 2015 (BST)). It can be thought that the initial structure(transition structure) in the input file deter the direction of pathway. The direction of path Two approaches were tries. The first approach is that last point on the IRC was optimised at HF/3-21G theory. The resultant electronic energy is -231.691667 cm-1.However, if the structure is not really similar to the transition structure, geometry with wrong minimum energy will be obtained. As for the approach 2, number of steps was increased from 50 to 100.but the electronic energy is still be same. It seems that the more steps is,The more subtle the process is. (Why is the process more subtle if you increase the number of allowed steps? Increasing the number of steps is of no use if your calculation has already converged. João (talk) 18:17, 14 April 2015 (BST))
Summary of Activation Energy
Following table summaries the activation energy corresponding to two different level of theory from the Gaussian. The activation energy of ' 'chair ' ' transition structure is lower, in comparison with that of ' 'boat ' ' transition structure. Therefore, kinetic product is formed if the reaction proceeds via ' 'chair' ' transition pathway. B3LYP/6-31G* level of theory can provide more accurate value as the value is closer to the experimental value. (You report energies for the transition state calculated with DFT, how do the geometries of these transition states compare with those of those calculated at he Hartree-Fock level? Dis you confirm it was a transition state at this level of theory? João (talk) 18:17, 14 April 2015 (BST))
| HF/3-21G | B3LYP/6-31G* | |||||
| Electronic Energy/Hartree | Sum of Electronic and Zero-point Energies/Hartree | Sum of Electronic and Thermal Energies/Hartree | Electronic Energy/Hartree | Sum of Electronic and Zero-point Energies/Hartree | Sum of Electronic and Thermal Energies/Hartree | |
| at 0 K | at 298.15 K | at 0 K | at 298.15 K | |||
| Chair TS | -231.619322 | -231.466700 | -231.461341 | -234.556983 | -234.414929 | -234.409009 |
| Boat TS | -231.60280249 | -231.450928 | -231.44299 | -234.543093 | -234.402342 | -234.396008 |
| Reactant (anti) | -231.69235 | -231.539542 | -231.532566 | -234.61171064 | -234.469219 | -234.461869 |
| HF/3-21G | HF/3-21G | B3LYP/6-31G* | B3LYP/6-31G* | Expt. | |
| 0 K | 298.15 K | 0 K | 298.15 K | 0 K | |
| Δ E(Chair) | 45.71 | 44.69 | 34.08 | 33.17 | 33.5± 0.5 |
| Δ E(Boat) | 55.61 | 56.21 | 43.06 | 41.97 | 44.7±2.0 |
Transition Structure of Diels Alder Reaction
Transition State for Prototype Reaction
The Diels Alder reaction is one type of percyclic reaction. The Characteristics of this reaction is concerted and stereospecific. In the first exercise, the reaction between the cis-butadiene and ethene were investigated by orbital interaction.4 Many dienes will have more than one conformations and the conformation can be interchanged by rotation around single bonds.41,3-butadiene will be interchanged from s-cis confromation to s-trans conformation by σ bond between the π bond.Among two conformations,only s-cis butadiene can take place.s-trans butadiene can not take place diels alder reaction because the terminal carbon of diene can not be close enough to react with ethene. Prototype reaction as a branch of diels Alder reaction must obey the rule which HOMO-LUMO interaction must have the same symmetry. In this section, semi-empirical AM1 was used for optimisation of Cis-butadene and ethene,and the HOMO and LUMO orbital for both of reactants are illustrated in table . The HOMO of ethene is symmetry while the LUMO of ethene is anti-symmetry with respective to the plane. In contrast ,the LUMO of butadiene is symmetry and the HOMO of butadiene is anti-symmetry with respective to plane.
| The type of orbital | Cis-butadiene | Ethene |
| HOMO | 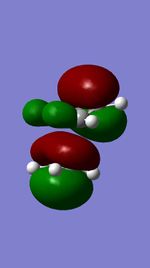 |

|
| LUMO |  |
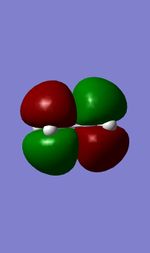
|
| Orbital | Relative Energy | |
| HOMO | 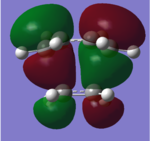 |
-0.32393 |
| LUMO | 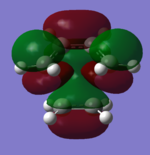 |
0.02315 |
| HF/6-31G* | IRC |
 |
|
An imaginary frequency (-956.04 cm-1) is given, which is relevant transition structure. The animation from table 11 is relevant to the reaction pathway.It illustrates that the formation of the two bonds is synchronous, which represents a interaction fragment with πp electrons with the fragment with πq electrons. The Woodward-Hoffman Rule is based on former one.4 According to the transition structure of prototype reaction,antisymmetric HOMO is generated from the orbital overlap of LUMO(ethene) and HOMO(cis-butadiene). Also, HOMO of ethene and LUMO(cis-butadiene) overlaps to form symmetric LUMO. According to the reference, C-C(sp3) bond length is 1.54 Å and C-C(sp2) is 1.47 Å. The van der waals radius of carbon is about 1.7 Å. The partial bond length found from the transition structure is about 2.28 Å which is much longer C-C(sp3) bond length, as the bond is partial formed (Why do you suggest there is a bond at all? How does the bond length compare with twice the van der Waals rarius for carbon? João (talk) 18:17, 14 April 2015 (BST)). Furthermore, bond length corresponding to the C=C bond and C-C is closer to 1.40 Å, which demonstrates that electrons are delocalised in the transition state.
Regioselectivity of the Diels Alder Reaction
The Diels Alder reaction will take place when the HOMO and LUMO are maximum overlapped. In most times,Diels-Alder reaction that between cyclohexdiene and maleic anhydride,there are two different possible transition states ,which are named the ' ' endo ' ' and ' ' exo ' '. Each transition structure will result in different stereoisomers. These names are due to the relationship in position between the carbonyl groups on the maleic anhydride and the double bond formed . If they are on the same side, the product is endo . If they are opposite to each other,the product is exo. The HOMO-LUMO interaction can illustrate the reaction pathway of Diels-Alder reaction and activation energy will be computed to confirm the kinetic product and thermodynamic product . Exo transition structure was optimised at HF/3-21G level of theory and 6-31G* level of theory, respectively, from the guess transition structure.' ' Endo ' ' transition structure was optimised by QST2 method at HF/3-21G level of theory and 6-31G* level of theory, respectively. The reaction path is performed by animation. The geometry corresponding to four orbital are all antisymmetrical,which proves reaction condition of Diels Alder reaction (Why? João (talk) 18:17, 14 April 2015 (BST)). HOMO, LUMO of transition structures are represented as follows.
| Exo | HOMO |  |
LUMO |  |
Transition Structure | 
|
| Endo | HOMO |  |
LUMO |  |
Transition Structure | 
|
Activation Energy of ' 'exo' ' and ' 'endo' ' product
| Cyclohexadiene/Hartrees | Maleic Anhydride/Hartrees | Endo Transition Structure/Hartrees | Exo Transition Structure/Hartrees | Activation Energy of Endo/Hartrees | Activation Energy of Exo/Hartrees | Activation Energy of Endo(kcal/Mol) | Activation Energy of Exo (kcal/Mol) | |
| 3-21G | -230.53967121 | -375.10351347 | -605.61036817 | -605.61036821 | 0.03281651 | 0.032811647 | 20.592655 | 20.5895743 |
| 6-31G* | -233.41587925 | -379.28953540 | -612.68339678 | -612.68339673 | 0.02201787 | 0.02201792 | 13.816412 | 13.917648 |
In comparison with exo structure, The endo product is less stable , which prefers in irreversible Diels-Alder-Reaction.Therefore, it is the kinetic product of the reaction,which can be proved by the activation energy of endo transition structure from the 6-31G*. The result computing from 3-21G level of theory gives a undesired result, which will be discussed in the future. It also can be explained by frontier molecular theory .Exo-product is the thermodynamics product,however, secondary orbital interaction(bond interaction between the carbonyl group of maleic anhydride with π bond at the back of cyclohexadiene) result in lower energy barrier of endo-transition state (Do you see that in your calculations? João (talk) 18:17, 14 April 2015 (BST)).Therefore,the reaction towards endo product can proceed faster. Furthermore,Exo transition structure is more strain because the -(C=O)-O-(C=O)- has more steric interaction with the remainder of system. Overall,this computational analysis is under ideal system and the solvent effect is neglected.6
Conclusion
All of sections were successfully run by the Guassian software . In the first section, the activation energy to ' 'chair or boat' ' transition state will be calculated and the value are involved in the expected range. However, the geometry with minimum energy from IRC was not found due to time limitation. As for the section about Diels Alder reaction, the result from 6-31G* level theory proves that endo product is preferred. However, the 3-21G method is unable to prove. Further effort are needed to figure out this problem.
Reference
1. P. Atkins,J.D.Paula,2010,Atkins' Physical Chemistry, 9th Edn,Oxford press,Oxford
2. B.W. Gung,Z.Zhou,R.A.Fouch,J.Am.Chem.Soc.,1995,117,pp 1783-1788
3. H. Sayin. Quantum Chemical Studies and Kinetics of Gas Reaction,2006,Aubrun,Alabama
4. J. Clayden,N. Greeves, S.Warren, Organic Chemistry,2rd Edn,Oxford Press,Oxford
5. Astle,W.H. Beyer,1984. CRC Handbook of Chemistry and Physics, 65th Edn, Inc. CRC Press, Boca Raton, FL
6. M.F.Ruiz-Lopez,X.Asseld,J.l.Garcia,J.A.Mayoral,L. Salvatella,J.Am.Chem.Soc.,1993,115,pp 8780-8787

