User:Tad10
I.The Cope Rearrangement Tutorial a)Molecule anti 1,5-hexadiene was drawn with energy of -231.6925 a.u and Ci symmetry. b)Molecule gauche 1.5-hexadiene was drawn, the energy expected to be higher than in anti molecule which is -231.0098 a.u with C2 symmetry. c)A structure that is similar to anti geometry will have a lower energy. d)The structure was compared to the table and it was Ci anti2. e)The energy of the optimized molecule compared to the one in the table is similar. f)The molecule was optimized at higher level. It now has the enery of -234.6117 a.u. It is not possible to compare the two energies since they are caculated based on two different levels/methods. g)Starting from the optimized b3LYP/6-31G* structure, Frequency was caculated and then: i) sym of electronic and zero points energies: -234.4571 a.u ii)-234.4514 a.u iii)-234.4504 a.u iv)-234.4863 a.u II. Optimize chair and boat TS a) Allyl fragment:
b)Chair structure:

c)Optimized chair structure:

III.The Diels-Alder cycloaddition i)Cis-butadiene: HOMO of cisbutadiene:

As we can see, HOMO of cis butadiene is antisymmetric. LUMO of cis butadiene:

ii)Transition State geometry for the prototype reaction was builded and optimized:

The two forming bonds comes together to react. HOMO of the transition state:

The HOMO of the transition state is antisymmetric.
iii)Regioselectivity of the Diel Alder Reaction -For exo product, the TS is :

The energy of exo form TS is -605.6034 au The bond length of the partialy form C-C bond is 2.26070 Amstrong. The HOMO of TS :

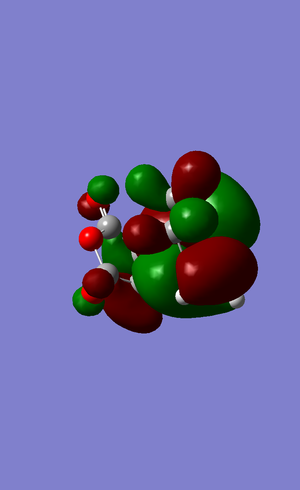
-For endo product, the TS is:

The energy of endo form TS is -605.6104 au which is lower as expected. The HOMO of TS :


-Both HOMOs are anti-symmetric. -As we can see from the two HOMOs of two TS, between the -(C=O)-O-(C=O)- fragment and the remainder system of endo TS there is an pi-orbital overlap whilst there is no such overlap in exo TS stucture.This is known as secondary orbital overlap effect. This is also the reason why endo product is formed instead of exo product. -The bond length of the partly form C-C bond is 2.26070 Amstrong. Typical C-C and C=C bond lengths are 1.54 and 1.47 Amstrong respectively. The van der Waals radius of the C atom is 1.7 A. So the C-C bondlength of the partly formed C-C in the TS is under van der Waals attraction force. -The HOMO of ethylene and the LUMO of butadiene are both s, they interact with each other. The LUMO of ethylene and the HOMO of butadiene are both a, these two orbital interact with each other.
