User:Se408
The Basic Techniques of Molecular Mechanics and Semi-Empirical Molecular Orbital Methods for Structural and Spectroscopic Evaluations
Introduction
Computational chemical anaylysis has been greatly significant in these days as technology is becoming powerful enough to perform the theoretical calculations. In addition to this, there are many programmes which strongly rely on chemical data base that is now ample to provide accurate predictions even on spectroscopic data. In this experiment, a combination of programmes was used, including ChemBio3D and Gaussian. The purpose of this experiment is to familiarise the programmes in order to apply them in real organic laboratories. This includes the minimization of chemical conformation, prediction of major isomeric product and calculations of spectroscopic data. In the end of this expeiriment, the validity of computational chemistry will be examined.
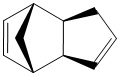
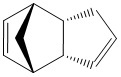
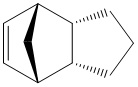
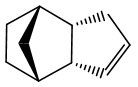
The Hydrogenation of Cyclopentadiene Dimer
|
Exo Dimer (1) |
Endo Dimer (2) |
Hydrogenated Endo Dimer (3) |
Hydrogenated Endo Dimer (4) |
|---|---|---|---|
|
|
|
Energies (kcal/mol) |
Exo Dimer (1) |
Endo Dimer (2) |
Hydrogenated Endo Dimer (3) |
Hydrogenated Endo Dimer (4) |
|---|---|---|---|---|
|
Stretch |
1.30001 |
1.2502 |
1.2659 |
1.1000 |
|
Bend |
20.5956 |
20.8555 |
19.8063 |
14.5121 |
|
Stretch-Bend |
-0.8463 |
-0.8334 |
-0.8276 |
-0.5478 |
|
Torsion |
7.6334 |
9.5050 |
10.8698 |
12.5072 |
|
Non 1,4 VdW |
-1.3992 |
-1.5182 |
-1.2207 |
-1.0509 |
|
1,4 VdW |
4.2246 |
4.3041 |
5.6394 |
4.5069 |
|
Dipole-Dipole |
0.3771 |
0.4455 |
0.1621 |
0.1407 |
|
TOTAL |
31.8853 |
34.0086 |
35.6953 |
31.1681 |
The table shows that exo product is more stable than endo product.
Hydrogenation of double bond in 6-membered ring results more thermomodynamically stable product than that of 5-membered ring.
Molecules 1 and 2
The above figure show the mechanism for the Diels-Alder raction for endo product.

The frontier orbitals are drawn to explain the for the endo product being the major product. As the two cyclopentadienes come together for the collision, we are able to see that symmetry is matched for bond formation. The two dashed lines indicate the formation of two sigma bonds. At the back of the diene, the symmetry of the orbitals is matched for bonding interactions. Although, these interactions do not directly lead to formations of any bonding, but these drive the transition state to resemble the endo product. The endo product is kinetically favoured over exo product.
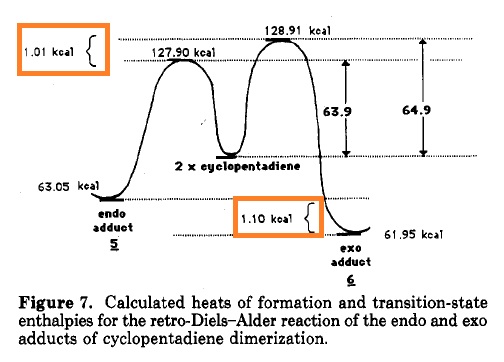
The above digram highlights that the reaction pathway to endo product is kinetically more favoured.
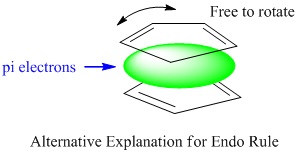
There is an alternative explanation for endo rule other than of frontier orbital approach. The through-space attractive HOMO/LUMO interactions between the two components results the transition state to look like a sandwich where the electrons are holding them together whilst allowing them free to rotate until the formation of sigma bonds. This vertical rotation greatly drive the formation of endo products.
The hydrogenation of the double bonds
It is expected that hydrogenation would preceed firstly via the double bond of the 6-membered ring followed by the 5-membered ring. It can be deduced from the energy values that the hydrogenation of double bond first occur at the 6-membered ring and then followed by the 5-membered ring. The great energy change from Molecule 2 to Molecule 4 is the relief of bending energy while to Molecule 3 does not feature that large energy. The kinetic product cannot be directly concluded from these energies. This should be accompanied by a series of experiment with reaction rate constant against the temperatures.
Stereochemistry of Nucleophilic Additions to a pyridium ring (NAD+ analogue)
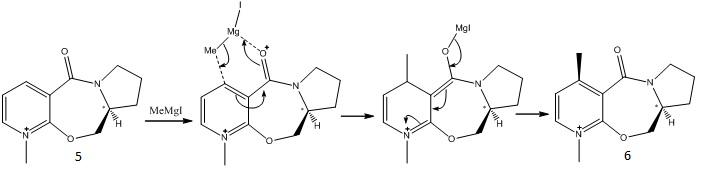
Molecules 5 and 6
|
|
|
|---|
The minimised energy for Molecule 5 was found to be 39.8642kcal/mol(by MM2) and 57.9231kcal/mol(MMFF94). The 7-membered ring was fairly rigid and only other fragments had significant effect on the total energy of the molecule.
After addition of the Grignard reagent (MeMgI), molecule 6 was formed. This reactions features a high steroselectivity where the Grignard reagent attacts from the top. This can be interpreted with the intermediate, in which the Mg chelates the oxygen atom (which always points up).Therefore, it is much easier for the methyl group to be added from top via a series of electron movement.
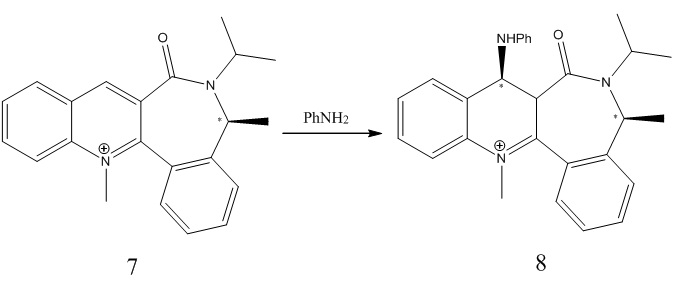
Molecules 7 and 8
|
|
|
|---|
The minimised energy for the ring was calculated by MM2, 62.4642kcal/mol with the carbonyl group pointing upwards (dihedral angle ~20 degrees). Therefore, the same mechanistical approach of the Mg metal is expected to happen here.
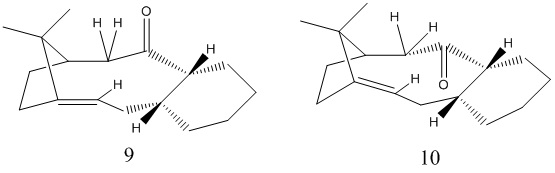
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
|
|
|
|---|
The total synthesis of Taxol proceeds via either intermediate 9 or 10.On standing, the compound isomerises into the alternative carbonyl isomer via atropisomerism. The stereochemistry of carbonyl addition is judged by which isomer is the most stable one. MM2 calculations reveals that Intermediate 9 has energy of 48.881kcal/mol and Intermediate has energy of 51.4286kcal/mol.On the other hand, MMFF94 calculations shows that Intermediate 9 has energy of 70.881kcal/mol and Intermediate has energy of 74.4286kcal/mol.
These are the details of energy compositions:
Intermediate 9
Stretch: 2.7066 Bend: 15.8737 Stretch-Bend: 0.4020 Torsion: 18.1901 Non-1,4 VDW: -1.0134 1,4 VDW: 12.5764 Dipole/Dipole: 0.1486
Intermediate 10
Stretch: 2.6887 Bend: 15.8369 Stretch-Bend: 0.3966 Torsion: 18.2591 Non-1,4 VDW: -1.1024 1,4 VDW: 12.6636 Dipole/Dipole: 0.1462
Scrutinisation of of the minimum energy conformations of intermediate 9 and 10 leads us to that cyclohexane ring possesses a boat conformation for intermediate 9 and a chair conformation of intermediate 10.
Modelling Using Semi-empirical Molecular Orbital Theory: Regioselective Addition of Dichlorocarbene
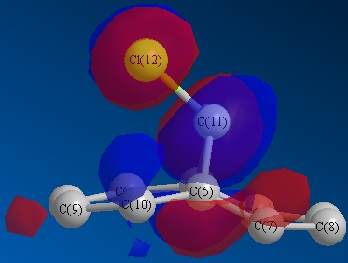
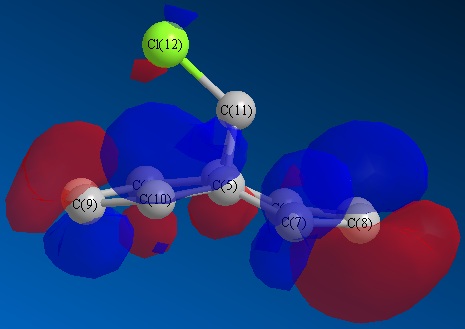
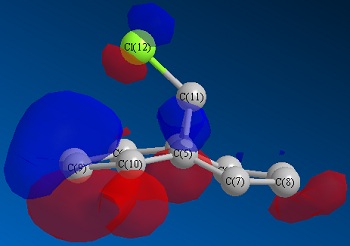
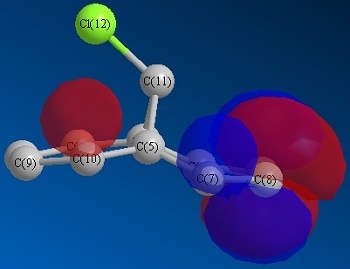
Optimisation via HF method led to above molecular orbitals.
Considering the orbital contribution to bond formation, as expected, the HOMO was concluded to be most amenable to attack of an electrophile. It can bee seen that the HOMO of the endo C=C double bond possesses a larger electron density in comparison to that of exo C=C double bond. This larger electron density imparts a greater nucleophilicity for endo C=C towards electrophiles. Another factor that enhances the reactivity of the endo c=c and stability of exo C=C is the interaction of the exo C=C pi orbitals with the antibonding orbitals of C-Cl bond. This lowers the total energy of exo C=C bond and make it less susceptible towards electrophilic reaction.
The addition of dichlorocarbone to this molecule 12 occurs on the endo C=C bond, even though there will be a greater steric repulsion of large chlorine atom. The following calculations reveal why this addition is still favoured.
Energy /kcalmol-1
Stretch 0.6156 Bend 4.7823 Stretch-Bend 0.0496 Torsion 7.6318 Non-1,4 VDW -1.1034 1,4 VDW 5.781 Dipole/Dipole 0.4125 Total Energy 18.2053 JMol
A disparity is observed between the hydrogen atom on the endo carbon double bond (2.8Å and 3.2Å). This contribute some effect to relieve the steric repulsion by the large chlorine atom. Therefore MM2 shows its ability to account for explaining this reaction.
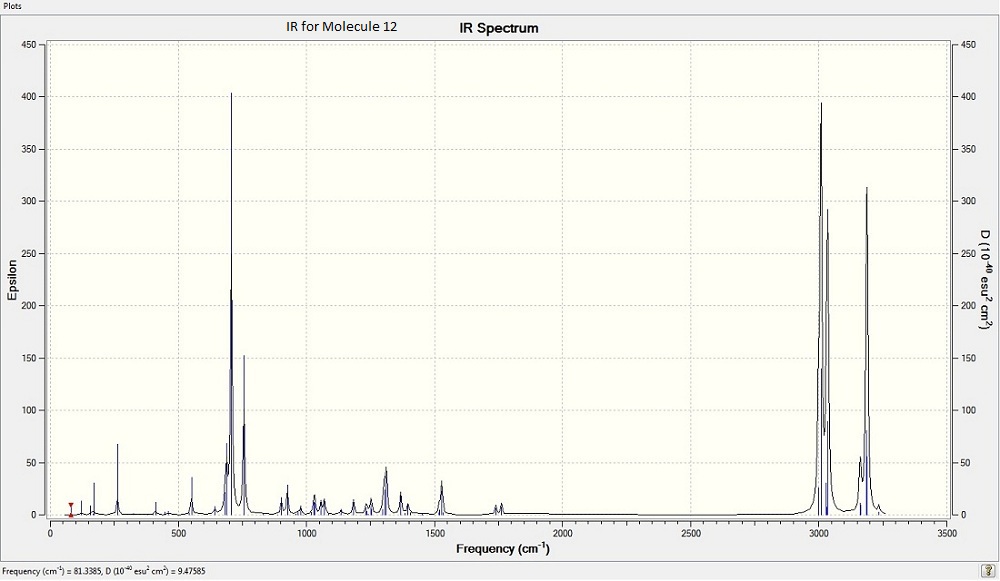
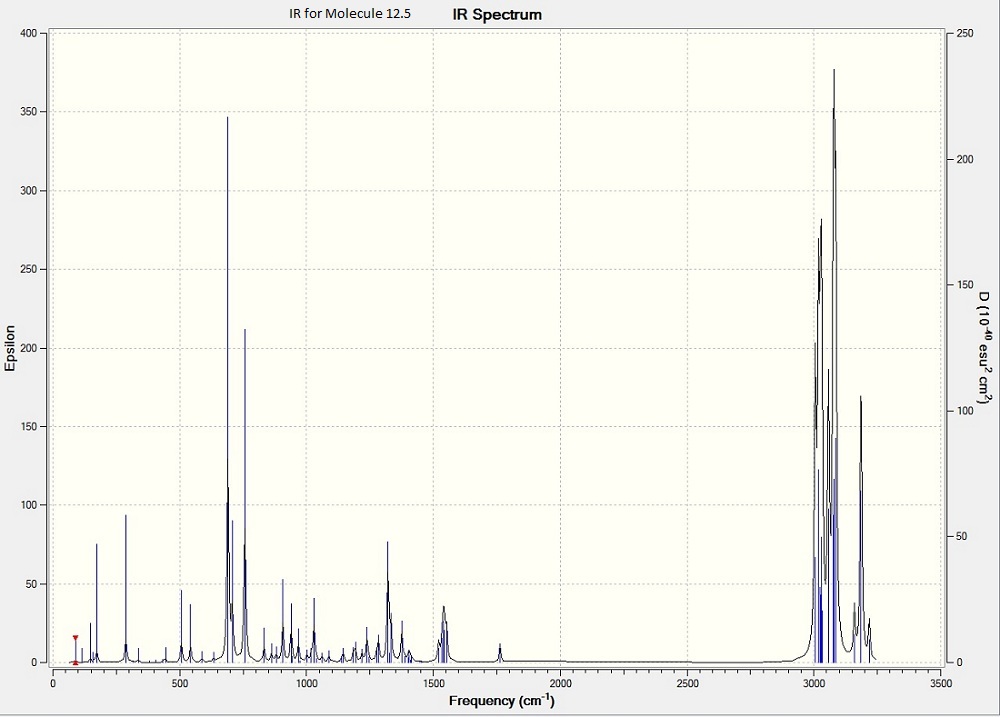
It is observed that the anti-alkene shows a lower stretching frequency than that of the syn-alkene.
Utilising the fact that the frequency of C=C bond represents the bond strength, it can be suggested that the exo C=C bond is weaker than the endon one.
C-Cl Stretch: 770.80cm−1 C=C Stretches: anti-alkene= 1740cm-1, syn-alkene= 1757cm-1
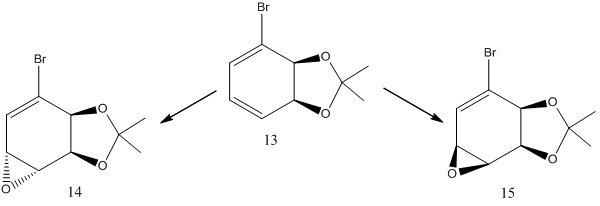
Structure based Mini project using DFT-based Molecular orbital methods
|
|
|
|---|

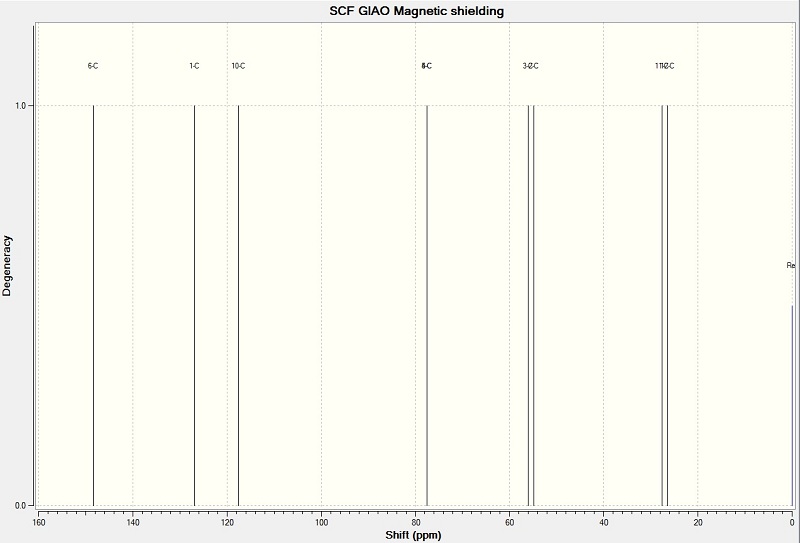
13C NMR Spectrum for Species 14 & 15 has the same chemical shifts
NMR Calculated[ppm]:
25.8 (s), 27.3 (s), 54.5 (s), 58.6 (s), 79.3 (s), 79.4 (s), 112.6 (s), 127.3 (s), 132.4* (s).
NMR Experimental [ppm]:
25.2 (s), 27.1 (s), 50.2 (s), 54.5 (s), 73.9 (s), 76.8 (s), 108.7 (s), 125.5 (s), 130.0 (s).
(*132.4ppm is the corrected for spin-orbit coupling erros. C-Br by -12ppm)
The calculated NMR data were close to the experimental data from the literature. This shows that the molecular mechanics method is a reasonable way to predict NMR values.
Above is the calculated 13C NMR
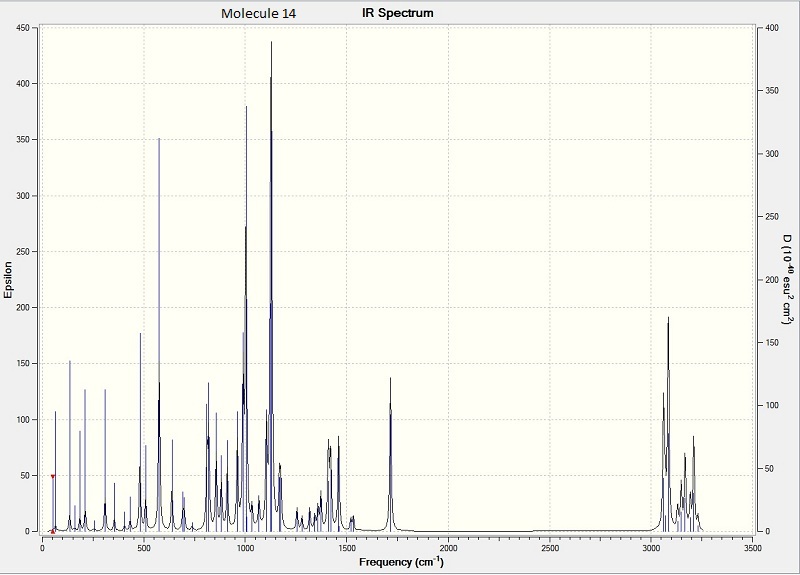
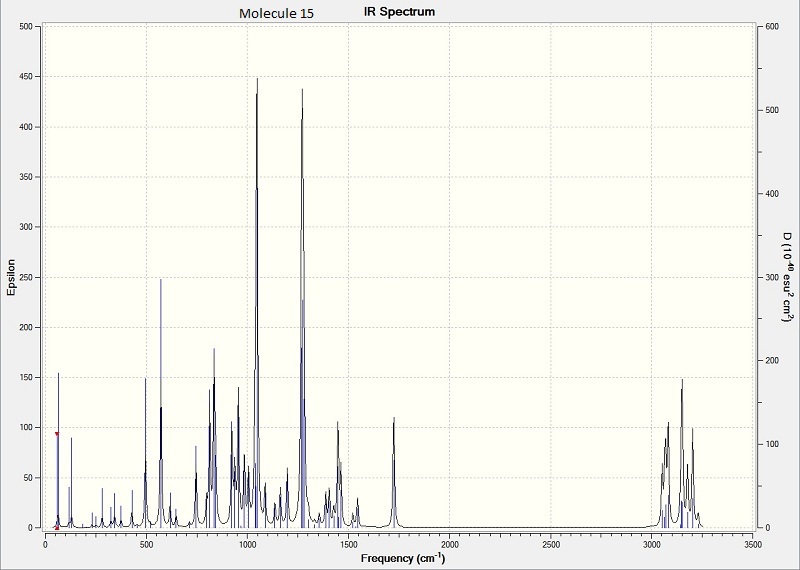
Above is the calculated IR for molecule 14
Above is the calculated IR for molecule 15IR spectra of the two compounds did not show a great difference.
In addition to NMR and IR, optical rotations were calculated:
For Molecule 14 = +103.34 degrees
For Molecule 15 = -84.35 degrees
The sign change shows that the modelled compounds were indeed stereoisomers of each other.
Reference
1. J. Clayden, N. Greeves, S. Warren, P. Wothers; "Organic Chemistry," p.916
2. A.G.Shultz, L.Flood, J.P.Springer, "J.Org.Chemistry," 1986, 51, 838
3. Borden, W. T. Chem. Rev. 1989, 89, 1095
4 "Further calculation explorations and predictions of hyperstable olefins": A.McEwen, P.Schleyer, J.Am.Chem.Soc., 1986, 108(14), 3951-3960
5. Brian Halton and Sarah G. G. Russell J. Org. Chem. 1991,56,pp. 5553-5556
6. Ba V. Nguyen, C. York, T. Hudlicky; "Chemoenzymatic Synthesis of Deoxyfluoroinositols," Tetrahedron, Vol. 53, No. 26, pp. 8807-88141, 1997.