User:Sc2108
Module 1: Structure and spectroscopy (Molecular mechanics/Molecular orbital theory)
Introduction
In the last 25 years, computer modelling has had a significant impact on science and technology (http://www.iupac.org/publications/pac/pdf/2005/pdf/7708x1345.pdf), especially chemistry where it is so much harder to illustrate atoms and bonds, never mind energy of reactions. Computational techniques were thus applied in this module in order to see how significant importance this has on chemistry in order to accurately model many aspects of structure and reactivity of the molecules. The main aims were to use molecular mechanics, semi-empirical molecular orbital theory and DFT method for calculations involving structure (energy,...) and spectroscopy. Firstly, molecular mechanics was used to predict the geometry and regioselectivity of the hydrogenation of cyclopentadiene dimer, stereochemistry of nucleophilic additions to a pyridinium ring and the conformation of a large ring ketone intermediate in Taxol formation. Semi-empirical methods were used to study the regioselectivity of the electrophilic carbenylation of a chloro-substituted bicyclic diene. Finally, DFT molecular orbital theory was used to inspect neighbouring group participation on the C-Cl and C=C stretching frequency of the previously investigated bicyclic diene. In the end, a mini project was conducted, investigating the two isomers from Diels Alder reaction and DFT molecular orbital theory was used to predict 13C NMR, IR and optical rotation.
Results and discussion
Isomerisation of cyclopentadiene
Cyclopentadiene dimerises into two possible isomers, endo and exo form. The endo dimer 2 is reported to be more favourable, although some of the exo dimer 1 is formed as well. An MM2 calculation was performed in ChemBio3D (12.0) in order to optimize geometries of the molecules and to obtain energies.
 |
 | |||
Table 1: Relative Energies of Exo isomer 1 and Endo isomer 2
| Energies/ kcal mol-1 | |||||||
| Stretch | Bend | Stretch-Bend | Torsion | Van der Waals | Dipole-Dipole | Total | |
| Exo isomer (1) | 1.28 | 20.57 | -0.83 | 7.67 | 4.24 | 0.38 | 31.88 |
| Endo isomer (2) | 1.25 | 20.86 | -0.83 | 9.50 | 4.30 | 0.44 | 34.02 |
It can be seen from the table above, that the exo product is lower in energy than the endo form by 2.14 kcal/mol. This suggests that endo dimer is kinetically favourable and exo dimer is thermodynamically favourable product. It is reported that endo product is the major product, therefore this means kinetically favourable product is a major product and that this Diels Alder reaction is kinetically controlled. Hence, this also suggests that the reaction is not reversible, which is common for Diels Alder reactions. Thus, the dimerisation of cyclopentadiene is kinetically controlled, giving the endo dimer as a major product and exo dimer as a minor, thermodynamic product.
Hydrogenation of cyclopentadiene
Hydrogenation of the endo dimer is reported to give two dihydro derivatives. Tetrahydro derivative, however, is formed only after a prolonged hydrogenation. Yet again, an MM2 calculation was carried on in order to optimize geometries and to obtain energies which are compared and summarised below.
 |
 | |||
Table 2: Relative Energies of Molecule 3 and Molecule 4
| Energies/ kcal mol-1 | |||||||
| Stretch | Bend | Stretch-Bend | Torsion | Van der Waals | Dipole-Dipole | Total | |
| Molecule 3 | 1.27 | 19.94 | -0.84 | 10.77 | 5.63 | 0.16 | 35.69 |
| Molecule 4 | 1.13 | 13.08 | -0.56 | 12.37 | 4.45 | 0.14 | 29.30 |
DISCUSSION!!
Stereochemistry of Nucleophilic Additions to a Pyridinium Ring (NAD+ analogue)
Reaction between derivative of prolinol and Grignard reagent
Reaction between aniline and PhNH2
Stereochemistry and Reactivity of Intermediate in Synthesis of Taxol
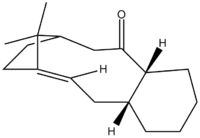
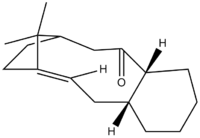
When synthesising an important anti-cancer drug - Taxol, there is a key intermediate, either (9) or (10) in the Figure X!!
. This was proposed by Paquette where oxygen is either pointing up (9) or down (10). On standing though, the compound isomerises between the two, which is described as atropoisomerism. Their properties were again studied in ChemBio3D, where MM2 and MMFF94 optimisations were produced in order to see which isomer is more stable.
Figure X: Possible intermediate 9 or 10 in Taxol synthesis
Since both molecules have a six-membered ring, the idea of boat/chair conformation has to be taken into account. In most occasions, a chair conformation is more stable that a boat. In order to see if this is true, both boat and chair conformations of the ring were examined, to obtain the structure with the lowest energy. At first, the optimisation gave a boat conformation, which was then manually dragged into a chair conformation and optimised again to yield a different energy. The optimised structures of both intermediates (9 and 10) can be seen below:
Table X: Relative Energies of Intermediates 9 and 10 obtained by MM2 optimisation
| Energies/ kcal mol-1 | |||||||
| Stretch | Bend | Stretch-Bend | Torsion | Van der Waals | Dipole-Dipole | Total | |
| Intermediate 9 - boat | 2.77 | 16.57 | 0.46 | 20.65 | 14.05 | 0.18 | 54.23 |
| Intermediate 9 - chair | 2.68 | 15.83 | 0.40 | 18.27 | 12.69 | 0.14 | 48.92 |
| Intermediate 10 - boat | 2.67 | 11.35 | 0.31 | 21.73 | 13.57 | -0.19 | 48.19 |
| Intermediate 10 - chair | 2.54 | 10.71 | 0.32 | 19.72 | 12.56 | -0.18 | 44.28 |
Table X+1: Relative Energies of Intermediates 9 and 10 obtained by MMFF94 optimisation
| Energies/ kcal mol-1 | |
| Total | |
| Intermediate 9 - boat | 77.92 |
| Intermediate 9 - chair | 70.55 |
| Intermediate 10 - boat | 66.31 |
| Intermediate 10 - chair | 60.56 |
DISCUSSION!!
Regioselective Addition of Dichlorocarbene
Part 1: Orbital control
DISCUSSION + reaction mechanism!!
Figure X: HOMO and HOMO-1 orbitals of 12 molecule
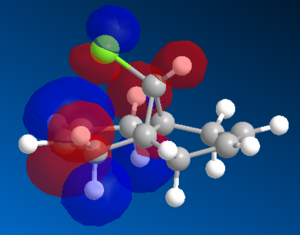 |
 |
DISCUSSION!!
Figure X: LUMO, LUMO+1 and LUMO+2 orbitals of 12 molecule
 |
 |
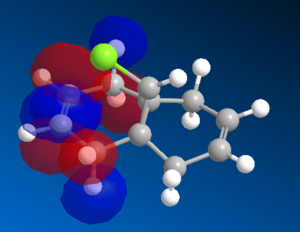 |
DISCUSSION!!
Part 2: Vibrational frequency
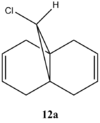 |
 | |||
DISCUSSION!!