User:Rew18
H-H-H System: Locating the Transition State
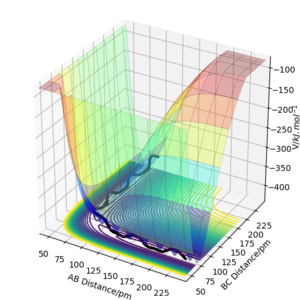
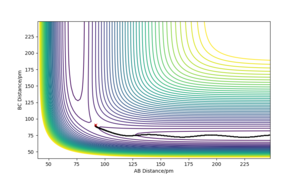
This is a potential energy surface diagram showing how the energy of the system varies as the distances between a hydrogen atom (A) and a hydrogen molecule with a bond distance of BC change. It is symmetrical along the line y=x as the reaction of 1/2 H2 and H2 is neither endothermic or exothermic. On a potential energy surface diagram the transition state is mathematically defined as the maximum on the minimum energy path where ∂V(ri)/∂ri=0 (the gradient of the potential is zero)The second derivative of potential energy is also positive in one direction and negative in an orthogonal direction. Pu12 (talk) 23:43, 26 June 2020 (BST) . The minimum energy path is the highlighted black line that links the reactants to the products What is plotted in the figure is a reactive trajectory, not a minimum energy path. You distinguish between the two below. João (talk) 18:50, 4 July 2020 (BST) , the transition state lies at the highest point of this line. The transition point can be identified by starting trajectories close to where one assumes the transition state to be and seeing whether the trajectory rolls towards the reactants or the products. If there is no roll the trajectory has been started at the transition state.
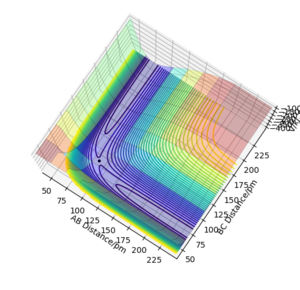
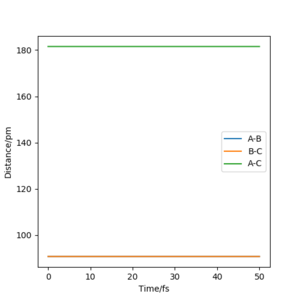
Here, the transition state has been located as there is no roll when the trajectory is started. Therefore for the reaction 1/2 H + H2, the transition state exists when the distance of AB and BC both equal 90.777pm and neither species has momentum. This can be further proven by a internuclear distance vs time plot as when both bond distances are set to 90.777pm and both species are given no momentum there are no forces acting along AB or BC, the internuclear distance of AB and BC are the same and constant and the internuclear distance of AC is constant. Good. Pu12 (talk) 23:43, 26 June 2020 (BST)
H-H-H System: Determining Successful Reaction Pathways
A minimum energy path (MEP) is a trajectory that corresponds to infinitely slow motion (achieved by setting the momentum to 0 at each step of the reaction) and is run from a point very close to the transition state, here it has been run from AB=91.777pm, BC=90.777pm, pAB=pBC=0. The trajectory just travels along the base of the potential energy diagram smoothly until the reaction has completed.
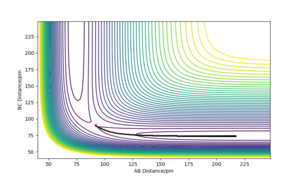
However MEPs are not an accurate representation of the reaction path It represents well the reaction path, what it does not represent is the actual motion of the atoms during a chemical reaction. João (talk) 18:50, 4 July 2020 (BST)
as a molecules motion is non-zero and constantly changing with the molecules having translational, vibrational and rotational degrees of freedom. When a dynamics calculation is run instead of an MEP the main difference in the plots is that the reaction path of the trajectory oscillates. This could be representative of the fact that the formed bond has vibrational energy, proving the dynamics calculation is a more accurate representation.
For the reaction to be successful the reactants must possess the energy to overcome the activation barrier. The following situations involve a collision of the atomic and molecular hydrogen with varying momenta and an AB and BC bond distance of 74pm and 200pm respectively .
| p1/ g.mol-1.pm.fs-1 | p2/ g.mol-1.pm.fs-1 | Etot/KJ.mol^-1 | Reactive? | Description of the dynamics | Illustration of the trajectory |
|---|---|---|---|---|---|
| -2.56 | -5.1 | -414.28 | Yes | A approaches BC with just enough energy to surpass the transition state, slowly forming AB + C What about vibrations? Pu12 (talk) 23:43, 26 June 2020 (BST) | 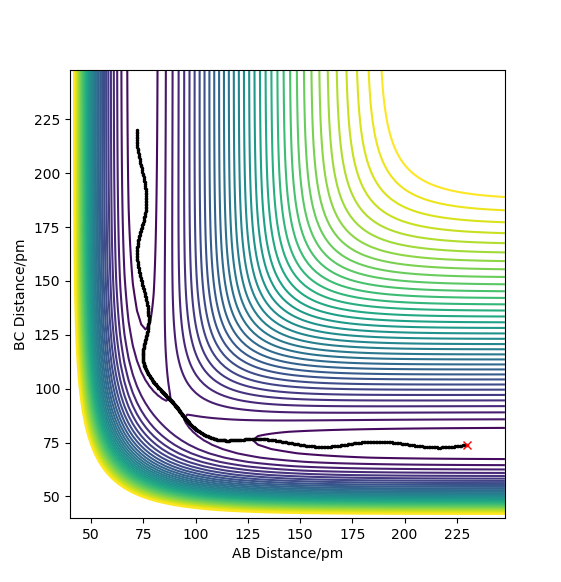
|
| -3.1 | -4.1 | -420.077 | No | A approaches BC but not without enough energy to reach the transition state. This results in the BC bond elongating but not breaking. | 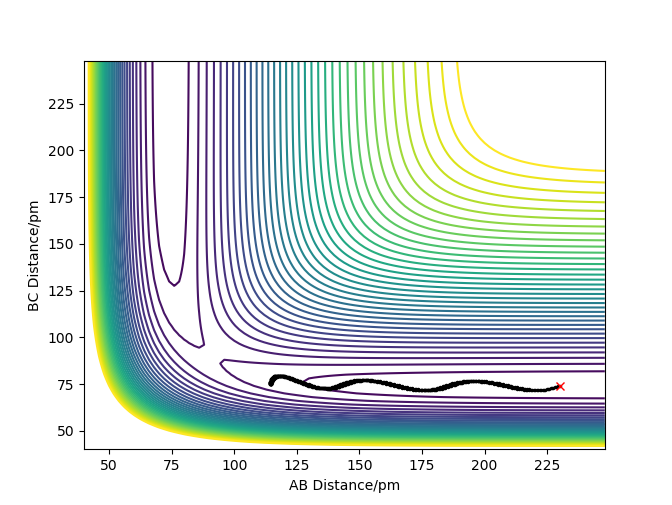
|
| -3.1 | -5.1 | -413.977 | Yes | A approaches BC in a similar fashion to the first reaction scenario however the system has more energy so the reaction is completed faster. | 
|
| -5.1 | -10.1 | -357.277 | No | A approaches BC with more than enough energy to react however once the transition state forms B oscillates between A and C eventually favouring to maintain its bond with C | 
|
| -5.1 | -10.6 | -349.477 | Yes | A approaches BC with enough energy to overcome the transition barrier. B oscillates between A and C before forming a bond with A | 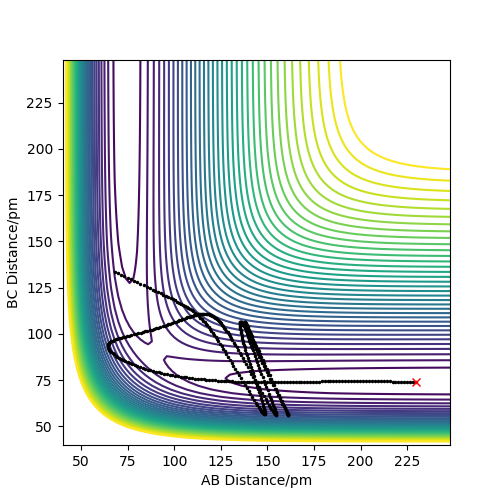
|
From this table we can conclude that for two species to react they must have enough energy to surpass the activation barrier. However just because they have enough energy and the transition state forms it does not mean the reaction will grant the products, the reactants may be returned.
H-H-H System: Transition State Theory
Transition State Theory uses 4 main assumptions to determine the rate of a reaction: [1]
1. Molecular systems that have surpassed the activation energy cannot turn back and form reactant molecules again
2. The distribution of energy in the system obeys Maxwell-Boltzmann distribution and when the system is in equilibrium, equilibrium theory can be used to determine the concentration of reactant molecules that have formed the transition state
3. Any reactant that comes close to reaching the transition state but does not quite reach it is simply treated as reactant
4. The system is treated classically, no tunneling through the activation energy barrier occurs
Clearly the calculations we have run are not based off of transition state theory as we previously had a scenario where the reactants possessed more than enough energy to form the transition state but the transition state broke down to afford the reactants, not the products. Transition state theory overestimates the reaction rate as the increase in rate brought about by the transition state not being able to afford the reactants again is greater than the reduction in rate we observe when we treat the system classically and don't account for quantum tunneling through the activation barrier.
F-H-H System: Reaction Energetics
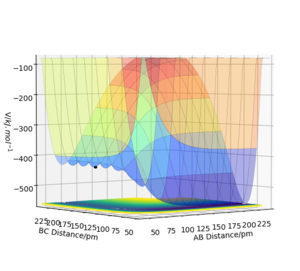
The system H(A)+H(B)F(C) is unlike H+H2 in the sense that it is asymmetric. This is reflected by its contour and surface plot as the transition state occurs when AB=74.5pm and BC=180.6pm and this point is no longer on the y=x axis. As the transition state is present when the BC bond is elongated it gives indication that in the transition state the H(A)-H(B) bond is stronger than the H(B)-F(C) bond. Using Hammonds postulate this tells you that the transition state resembles the products more than the reactants and that the reaction is endothermic. You wouldn't explain this using Hammond's postulate, rather you would explain Hammonds postulate using these models. A statement about the surface plot showing how energy changes, therefore endothermic, is enough. Pu12 (talk) 23:43, 26 June 2020 (BST)
To determine the activation energy of this reaction the energy of the transition state was determined from the surface plot (-433.981 KJ.mol^-1). A surface plot of the reactants was then plotted with an AB distance of 500pm and a BC distance of a literature value for HF bond length (91.7pm) [2] which gave the energy of the reactants to be -560.672KJmol^-1. The energy of the reactants was then subtracted from the energy of the transition state to obtain an activation energy of 126.691KJmol^-1.

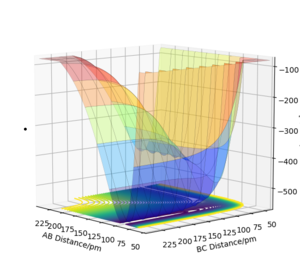
The system F(A)+H(B)H(C) is also asymmetric, the transition state occurs when AB=180.6pm and BC=74.5pm. Hammonds postulate again can be used to determine whether it is an endo or exothermic reaction. Its contour and surface plot shows the transition state lies towards an elongated AB distance representing the fact that in the transition state the bond between the hydrogen species is stronger than the FH bond. This means that the transition state more closely resembles the reactants so the reaction is exothermic.
To determine the activation energy of this reaction the energy of the transition state was determined from the contour plot (-433.981KJmol^-1). A surface plot of the reactants was then plotted with a AB distance of 500pm and a BC distance of a literature HH bond length (74.1pm) [3] which gave the energy of the reactants to be -435.097KJmol^-1. The energy of the reactants was then subtracted from the energy of the transition state to obtain an activation energy of -1.116KJmol^-1

As F + H2 is exothermic the system gives off energy and due to the principle of conservation of energy it means the newly formed H-F bond must be stronger than the old H-H bond as bond breaking is exothermic. Conversely, H + HF is endothermic, the newly formed H-H bond must be weaker than the reactant H-F bond which it is.Good. Pu12 (talk) 23:43, 26 June 2020 (BST)
F-H-H System: Reaction Dynamics
For the reaction of F(A) and H(B)-H(C) when the distances and momenta of AB and BC are 200pm and -2.12gmol^-1.pm.fs^-1 and 74.5pm and -3.88gmol^-1.pm.fs^-1 respectively there is a succesful reaction where a F-H bond is formed.

The plot of momenta vs time for this reaction shows that the product HF bond has much more momentum than the reactant HH bond. This could be because as the reaction is exothermic, the energy released from the system is released as vibrionic motion in the product. This could be proven experimentally with the use of IR. The energy of the product FH bond would be measured in an IR spectrometer and the value converted from wavenumbers to KJ.mol^-1. If this value is equal to the enthalpy of the reaction minus the initial energy of both reactant species this explanation would be proven correct You could use IR do detecct the vibrational energy change, but your discussion assumes that all of the enthalpy change is expressed as vibrations, you could simply get more transnational energy as heat that wouldn't necessarily show up on IR. Pu12 (talk) 23:43, 26 June 2020 (BST) .
Polanyi's Empirical Rules
Polanyi used potential energy surfaces like the ones used in this report to determine the effect of how early a reactions transition state is on the energy distribution of the products [4]. He determined that an earlier transition state tends to favour the vibrational excitation of the product molecule whereas a later transition state tends to lead to little vibrational excitation of the product molecule. In the last section the reaction of F + H2 was modelled and the resulting F-H species had high vibrational motion. Using Polanyi's empirical rules this would suggest that this reaction therefore had an early transition state. This is correct as this reaction is exothermic as the transition state resembles the reactants more than the products.This question is about the efficiency of the reaction, Polanyi's rules show how different energy types are more effective at causing reactions depending on their transition state position. Again, the Hammond postulate shouldn't be used to explain these models.Pu12 (talk) 00:35, 27 June 2020 (BST)
Bibliography
[1] - J. I. Steinfeld, J. S. Francisco, W. L. Hase Chemical Kinetic and Dynamics 2nd ed., Chapter 10, Prentice-Hall, 1998
[2] - (https://cccbdb.nist.gov/exp2x.asp?casno=7664393&charge=0)
[3] - https://cccbdb.nist.gov/exp2x.asp?casno=1333740&charge=0
[4]- J. I. Steinfeld, J. S. Francisco, W. L. Hase Chemical Kinetic and Dynamics 2nd ed., Chapter 4, Prentice-Hall, 1998
