User:Qz3617
Molecule of NH3
Molecule information
N-H Distance:1.01798Å
H-N-H Bond angle:105.741°
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au):-56.55776873
The point group of the molecule:C3V
Item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Jmol file
AMMONIA MOLECULE |
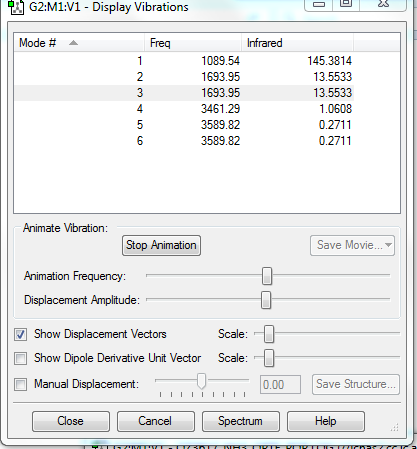
Screen shot of vibrations of NH3
1.How many modes do you expect from the 3N-6 rule?
- 3*4-6=6.
2.Which modes are degenerate? - Modes 2and3 are degenerate. Modes 5and6 are degenerate.
3.Which modes are "bending" vibrations and which are "bond stretch" vibrations? - Modes 1and2and3 are bending vibrations. Modes 4and5and6 are bond stretch vibrations.
4.Which mode is highly symmetric? -Mode 4.
5.One mode is known as the "umbrella" mode, which one is this? -Mode 1.
6.How many bands would you expect to see in an experimental spectrum of gaseous ammonia? -4 bands.
The charge on the N-atom is -1.125. The charge on the H-atom is 0.375. The nitrogen is more electronegative than hydrogen. So the charge on N should be negative and charge on H should be positive.
Molecule of H2
Molecule information
H-H Distance:0.74279Å
H-H Bond angle:180°
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au):-1.17853936
The point group of the molecule:DinfH
Item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Jmol file
HYDROGEN MOLECULE |
Screen shot of vibrations of H2
Molecule of N2
Molecule information
N-N Distance:1.0550Å
N-N Bond angle:180°
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au):-109.52412868
The point group of the molecule:DinfH
Item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
Jmol file
NITROGEN MOLECULE |
Screen shot of vibrations of N2
Energy of the reaction N2 + 3H2-> 2NH3
E(NH3)=-56.55776873au 2*E(NH3)=-113.11553746au E(N2)=-109.52412868au E(H2)=-1.17853936au 3*E(H2)=-3.53561808au ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907au=-146.48KJ/mol
Ammonia is more stable. Because the energy for ammonia is the lowest.
Molecule of F2
Molecule information
F-F Distance:1.40281Å
F-F Bond angle:180°
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au):-199.49825218
The point group of the molecule:DinfH
Item table
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
Jmol file
FLORINE MOLECULE |
Screen shot of vibrations of F2
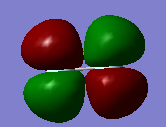
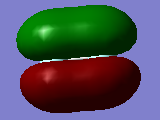
Molecular orbitals of F2
The first graph is the 2s boding orbital. Its energy is -1.33659.
The second graph is the 2s anti-bonding orbital. Its energy is -1.09047.
The third graph is the 2p bonding orbital. Its energy is -0.58753.
The fourth graph is the 2p anti-bonding orbital.Its energy is -0.39190.
The fifth graph is the pi bonding orbital.Its energy is -0.52332.
All of these five orbitals are in the HOMO region and they are occupied.Also, anti-bonding orbital has higher energy than the bonding orbital.
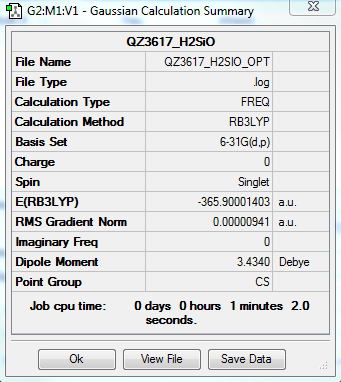
Extra molecule H2SiO
Molecule information
H-Si Distance:1.48652Å
Si-O Distance:1.53172Å
H-Si-H Bond angle:105.741°
O-Si-H Bond angle:124.158°
Calculation method:RB3LYP
Basis set:6-31G(d.p)
Final energy E(RB3LYP) in atomic units (au):-365.90001403
The point group of the molecule: CS
Item table
Item Value Threshold Converged? Maximum Force 0.000539 0.000450 NO RMS Force 0.000222 0.000300 YES Maximum Displacement 0.001573 0.001800 YES RMS Displacement 0.001153 0.001200 YES
Jmol file
H2SiO MOLECULE |