User:Mp4114
Introduction
By anlysising potential energy surfaces of different species involved in a reaction, an accurate and indepth knowledge of a tranistion state can be obtained. Throughout the experiments three main methods of locating transition states were tested. Each method had benefits and draw backs. Method one was very fast but not very reliable as it required an in depth knowledge of the transition state. The method involved making a guess structure for the transition state and immediately optimising it and carrying out frequency calculations. This could have some use for very simple molecules however the molecules involved in this experiment were too complex therefore this method was seldom used. The second method was more accurate and was used for experiment one and two. It involved freezing the distances between the molecules before a transition state was formed and then optimising. The optimised reactants were then used to find a transition state. The transition states for experiments 1 and 2 were known therefore this method was particularly useful. The final method involved working back from the product towards the transition state by altering angles and bonds. This was useful for experiment 3 as the molecules were more complex and thus the transition state became difficult to deduce before hand. PM6 and B3LYP optimisation were used during the reactions. PM6 proved unreliable but was very quick, B3LYP was very reliable but too slow and therefore impractical to use throughout the experiment. IRC calculations were also carried out in order to prove if the tranistion structure obtained was accurate by illustrating the reaction progression from reactants through to products.2
Nf710 (talk) 15:08, 4 November 2016 (UTC) You should have defined what a TS and minimum is and how to define it.
Exercise 1: Reaction of Butadiene with Ethylene
All species during this reaction were PM6 optimised. The orbitals of the transition state and reactants were studied in order to determine which species transferred electrons during the transition state formation. Alterations in bond length also provided insight into the changes in orbital hybridisation as the reaction progressed.

MO anlysis
Analysing the MOs of the transition state and reactants provided insight into which specific orbitals interacted during the reaction. The HOMO of the transition state shows that it was comprised of the of the HOMO of the butadiene molecule and LUMO of ethene molecule. The MO appears to have fairly simialr contributions from each reactant (weighting coefficients are similar), this indictaes that the two interacting orbitals are also close in energy. However it can be seen that the orbital contribution from the butadiene molecule is slight larger. The overlap intergral between these two orbitals would also be a non - zero value and the overlap integral between the orbitals that do not interact would be 0.
The HOMO and LUMO of the two interacting molecules are shown below. It can be seen that these two orbitals are both gerade. The fact that the two interacting orbitals are of the same symmetry highlights another important pre-requisite to orbital mixing; both orbitals have to be of the same symmetry. Orbitals that do not interact would be of different symmetry. The overlap intergral between two orbitals of the same symmetry would be a non - zero value and the overlap integral between orbital of different symmetry would be 0.5
 |
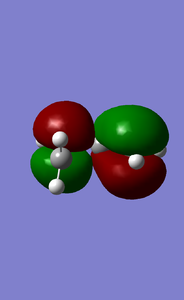 |
 |
|---|
These pieces of information enabled me to construct my own MO diagram of the reaction:
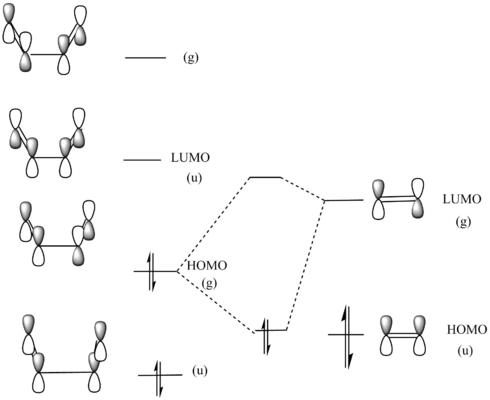
The HOMO of ethene and LUMO of butadiene are also of the same symmetry however they are too far apart in energy for there to be a considerable bonding interaction thus the reaction can be described as normal electron demand.1
Nf710 (talk) 15:15, 4 November 2016 (UTC) they would still interact somewhat but the orbital ordering wouldnt change so it would stay normal demand
Bond length analysis
Analysis of the changes in C-C bond lengths throughout the reaction provides insight into how the hybridisation of the orbitals changes as the reaction progresses.
| Reactant bond | C-C bond length (Å) | Transition State | C-C bond length (Å) | Products | C-C bond length (Å) |
|---|---|---|---|---|---|
| Ethene | 1.32731 | Terminal carbons | 2.13182 | sp2 - sp2 | 1.37980 |
| Butadiene (single) | 1.47070 | Butadiene component (double) | 1.37980 | sp3 - sp3 | 1.41113 |
| Butadiene (double) | 1.33346 | Butadiene component (single) | 1.41113 | sp2 - sp3 | 1.50033 |
| Ethene component | 1.38175 |
The length of the ethene C-C bond increases when forming the transition state. This increases in size illustrates how the sp2 hybridised carbons gradually gain more p character as the transition state forms and then finally a saturated bond with the butadiene molecule forms as the product is developed. Following the change in size of reactants bonds enables us to also follow changes in hybridisation as the reaction progresses. The distance between the two terminal carbons in the transition state is 2.13182 Å, the combined Van Deer Waals radius of two carbon atoms is 3.4 Å and the the average size of an sp3 - sp3 C-C bond is 1.54 Å. The fact that the distance between the two terminal atoms in the transition state lies in between the Van Deer Waals radius and sp3 - sp3 C-C bond illustrates how the interaction that exists between the two reactants in the transition state can not be considered a complete bond but there is certainly some form of bonding interaction.
Vibrational modes
In the tranistion state the bonding that occurs between the terminal carbon atoms of the two components occurs in a synchronous motion. This is illustrated by the vibration at the transition state.
The animation below illustrates the asynchronous motion of the vibration with the lowest positive value.

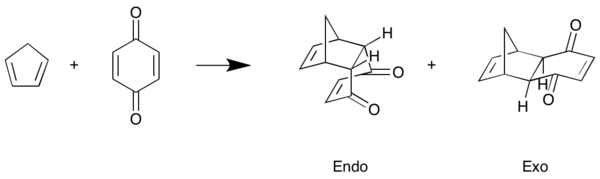
Exercise 2: Reaction of Benzoquinone with Cyclopentadiene
Changing the position of the two molecules before they reacted resulted in the formation of two different products. The endo - product and the exo - product. During this experiment the reactants, transition state and products were all initially PM6 optimised and then (to improve the accuracy of the calculations) were B3LYP/6-31G(d) optimised. This provided a reliable platform to investigate orbital interactions and the thermodynamics of the reaction in order to determine what controlled development of the exo and endo product.
MO analysis
The MOs of the endo and exo transtion states and of the optimised reactants indicated the movement of electrons during the reactions and informs us as to whether the reactions are normal electron demand or inverse electron demand. It can be seen from the table of MOs below that the that the HOMO of diene and the LUMO of the dieneophile interact in-phase during the endo reaction to form the transition state, thus the endo reaction is an example of normal electron demand.
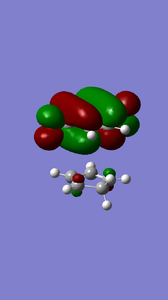 |
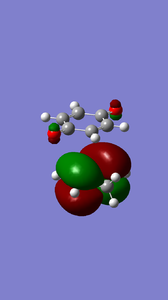 |
 |
|---|
Formation of the exo-product also occurs via normal electron demand as can be observed by the in-phase interaction of the diene HOMO and dieneophile LUMO.
(What about the formation of the LUMO of the TSs? Tam10 (talk) 11:25, 31 October 2016 (UTC))
 |
 |
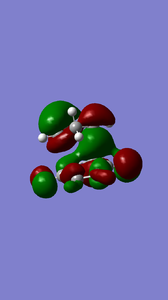 |
|---|
(You've lost symmetry in the exo TS. You should check that it's converged properly Tam10 (talk) 11:25, 31 October 2016 (UTC))
The presence of an enolate group of Benzoquinone is electron withdrawing, this is further evidence that the reaction occurs via normal electron demand. Benzoquinone contains π orbitals in the double bond and carbonyl group, cyclopentadiene contains π bonds present in the double bonds. During the reaction in the endo configuration there is a π - π interaction, this is due to the endo configuration having the correct positioning for the orbitals to overlap and is described as a secondary orbital effect. The result of this interaction is that the endo transition state is lowered in energy. The effect can be observed in the HOMO of the endo transition state, there is a clear in - phase interaction between the orbitals on the different unsaturated groups. The exo reactants are not in the correct position for this effect to occur. The unsaturated bonds of the reactants are not in the correct orientation for there to be secondary orbital interactions. This incorrect orbital oreintation can also be observed in the HOMO of the exo transition state. 4

3
Nf710 (talk) 15:35, 4 November 2016 (UTC) Awesome drawing explainingg SOO.
Thermodynamics
The energy values of the reactants, transition state and products provide quantitative evidence of the presence of the secondary orbital effect and thus identifies that the endo product is the kinetic product and the exo product is the thermodynamic product.
| Exo | Energy (KJmol-1) | Endo | Energy (KJmol-1) |
|---|---|---|---|
| Reactant | -1510762.2375 | Reactant | -1510762.22962 |
| T.S. | -1510665.36441 | T.S. | -1510670.426379 |
| Products | -1510788.14593 | Products | -1510783.84011 |
| ∆G‡ | 96.87309 | ∆G‡ | 91.803241 |
From the table it can be seen that the exo transition state had a higher ∆G‡ yet a lower energy product and is thus the thermodynamic product. The endo transition state had a lower ∆G‡ yet a high energy product and is thus the kinetic product. The endo product would form faster and irreversibly at low temperatures and the thermodynamic product would form reversibly at higher temperatures.
Nf710 (talk) 15:34, 4 November 2016 (UTC) Your conclusions for the endo are correct however for the exo, you have said that it is the thermodynamic product when it is not. Your barrier is also off for it, so I assume your product is not correct.
Exercise 3: Diels-Alder vs Cheletropic
This experiment involved investigating the different possible reaction paths of the cycloaddition reaction between o-Xylylene and SO2. Depending on the orientation of the reactants two different products are possible via different transition states. The diels-alder reaction will produce a product with both an C-O bond and S-C bond integrated into the molecule whereas the cheletropic reaction produces two C-S bonds. The diels-alder reaction will also yeild both an exo and endo product depending on the approach trajectory of the S02 molecule. All species were PM6 optimised during the experiment. IRC calculations and data on energy was collection in order to provide insight into the activation energies and thermodynamics of the reactions.
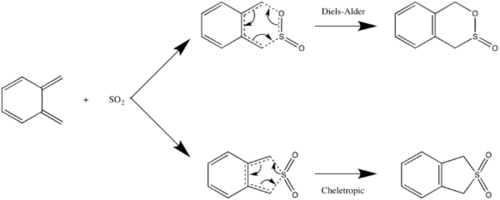
IRC calculations
The results of the IRC calculations are displayed below. A .gif file is provided along with a graphical illustration of the reaction pathway.
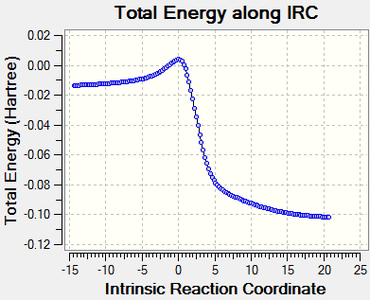 |
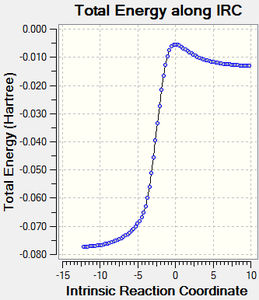 |
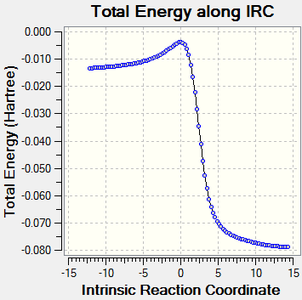 |
|---|
Log file link
Log file link
Log file link
Thermodynamics
The xylene species is highly reactive. As the reaction progresses a delocalised pi bond gradually forms in the six-membered ring. This delocalised pi bond is very stable and thus favorable making xylene an unstable reactant. The images below taken from the IRC calculation of the endo diels - alder reaction illustrates this process.
 |
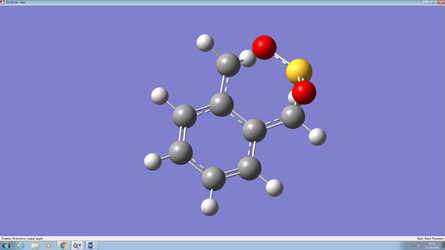 |
 |
|---|
(A nice, clear explanation of thermodynamic instability Tam10 (talk) 11:42, 31 October 2016 (UTC))
In order to find the reaction energies and activation energies of each reaction the sum of electronic and thermal free energies was obtained from the .log files of each species. This data was used to produce the information shown in the table below.
| Exo Diels - Alder | Energy (KJmol-1) | Endo Diels - Alder | Energy (KJmol-1) | Chelotropic | Energy (KJmol-1) |
|---|---|---|---|---|---|
| ∆G‡ | 65.90006 | ∆G‡ | 59.3363 | ∆G‡ | 73.7056671 |
| Reaction energy | 159.415121 | Reaction energy | 133.66159 | Reaction energy | 186.38426 |
(Use negative numbers for the reaction energies to show energy is being released. Check the exo reaction energy Tam10 (talk) 11:42, 31 October 2016 (UTC))
It is clear from the results that the endo prodcut is again the kinetic product of the diels-alder reaction. This is also due to the presence of secondary orbital effects. The endo product had the lower ∆G‡ and lower final energy thus defining it as the kinetic product. The exo product is thus the thermodynamic product. The results show that the chelotropic reaction had a comparably large ∆G‡ and reaction energy energy. The high ∆G‡ is due to the formation of a five memembered ring in the transition state which is less energetically favorable than a six memembered ring due to angualar strain. The cheltropic reaction has the largest reaction energy due to the presence of a sulfonyl group in the prodcut. This group contains two double sulfur and oxygen bonds which has a large bond enthlapy thus stabailising the product. Below are reaction profiles illustrating the different thermodynamic quantaties of each reaction..
 |
 |
 |
|---|
(Strange reaction coordinate diagram. Wouldn't it be easier to just draw lines between the energy levels? It would make it clearer if they were also on the same diagram Tam10 (talk) 11:42, 31 October 2016 (UTC))
Conclusion
The experiment illustrated that methods two and three were highly valuable when attempting to find an accurate transition state structure. Method one was redundant during the reaction as the simplicity lead to hugely inaccurate results. The results illustrate that there are many cdonsiderations forming an accurate transition state structure. Not only must the reactants be of the correct energy but also must have optimal distances and angles before reactions occur. Sterics and secondary orbital contributions play a very important role in transition state energy. PM6 optmisation is quick but is highly inaccurate at times and B3LYP is a necessity when accurately analysing energy values.
References
1. J. Clayden, N. Greeves and S. Warren, Organic Chemistry, Oxford University Press, 2nd edt.
2. Imperial college computational tutorial, https://wiki.ch.ic.ac.uk/wiki/index.php?title=Mod:ts_tutorial, accessed 20/10/2016
3. Image taken from http://pms.iitk.ernet.in/wiki/index.php/Pericyclic_Reactions_Electrocyclizations,_Cycloadditions_and_Sigmatropic_Rearrangements 20/10/2016
4. Raphael M. Ottenbrite Raphael M. Ottenbrite , Secondary Orbital Interactions Determining Regioselectivity
in the Lewis Acid Catalyzed Diels-Alder Reaction. I1 E. I. du Pont de Nemours and Company, Analytical Research Group, Spruance Plant, Richmond, Virginia 23261
5. 3. Shriver and Atkins’, Inorganic Chemistry, Oxford University Press, 5th edt.
