User:Mc2916
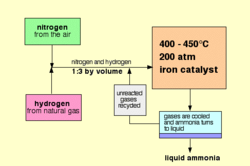
Introduction of Haber-Bosch process
Haber-Bosch process is a reaction that commonly used in industry to make ammonia by Nitrogen gas and Hydrogen gas. K2O, CaO, SiO2, and Al2O3 are usually used as catalyst to speed up the reaction. The reaction equation is as shown below
N2 + 3 H2 → 2 NH3
NH3
Information about NH3 molecular
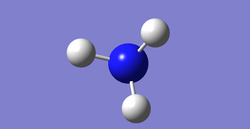
NH3 optimisation
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -56.55776873 |
| Point Group | C3V |
The bond length of NH3=101.2 Bond angle of NH3=106.7
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986295D-10
NH3 after optimisation |
The optimisation file is liked to here
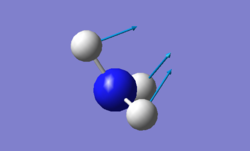
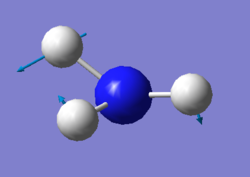
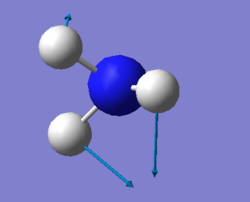
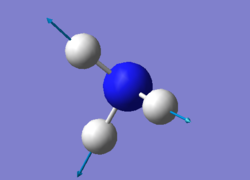
vibration of NH3
 |
 |
 |
 |
 |
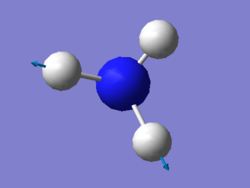 |
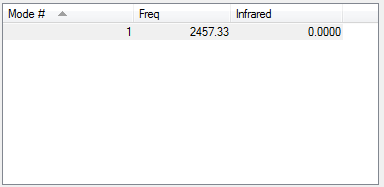
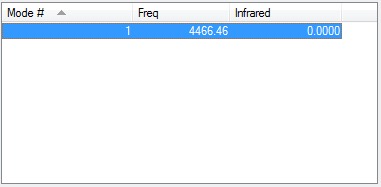
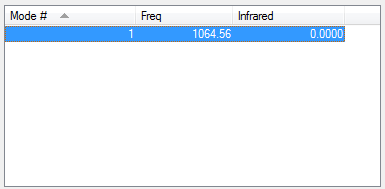
For NH3 molecule, 6 vibration modes are expected. In these 6 modes, mode1, mode2 and mode3 are banding vibrations while the rest 3 modes are stretching vibrations. Some modes have the special features, mode4 is highly symmetric, and mode1 is know as umbrella mode. Furthermore, some modes show similar energy levels, mode2 and mode3 are degenerate, mode5 and mode6 are degenerate. Four different energies are obtained from the vibration modes, therefore four bands are expected to be seen. However, in experiment only 3 peaks are observed in the spectra. The reason for this observation is due to only vibration that contains dipole can be detected. Among the four vibrations, mode4 doesn't have the dipole, then the peak is not shown on the spectra.

charge distribution of NH3
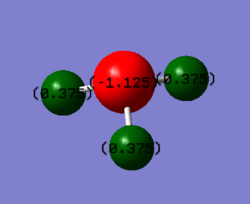
The charge of N is expected to be negative due to its high electronegativity, and H is expected to be positive dur to its low electronegativity.
N2
Information about N2 molecular

| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -109.52412868 |
| Point Group | D*H |
The bond length of N2=1.10 Bond angle of NH3=180
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-1.025171D-15
H2 after optimisation |
The optimisation file is liked to here
vibration of N2
H2
Information about H2 molecular
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -1.17853936 |
| Point Group | D*H |
The bond length of N2=0.74 Bond angle of NH3=180
Item Value Threshold Converged? Maximum Force 0.000039 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000052 0.001800 YES RMS Displacement 0.000073 0.001200 YES Predicted change in Energy=-2.025007D-09
H2 after optimisation |
The optimisation file is liked to here
vibration of H2
Haber-Bosch reaction energy calculation
E(NH3)=-56.55776873 2*E(NH3)=-113.11553746 E(N2)=-109.52412868 E(H2)=-1.17853936 3*E(H2)=-3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557907 Final ΔE is -0.06 KJ
Since the result is negative, it is an exothermic reaction, the equilibrium is prefer to move to the right. In a conclusion, it is more stable in ammonia product.
F2
Information about F2 molecular

| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| E(RB3LYP) | -199.49825220 |
| Point Group | D*H |
The bond length of N2=1.42 Bond angle of NH3=180
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.347176D-13
F2 after optimisation |
The optimisation file is liked to here
vibration of F2
Charge distribution of F2
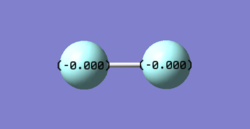
The charges on both F atom are zero due to they have the same electronegativity.
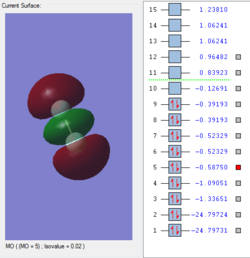
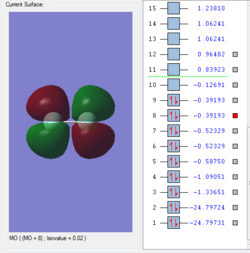
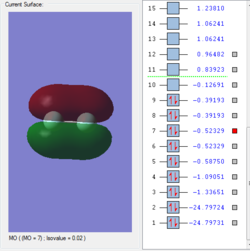
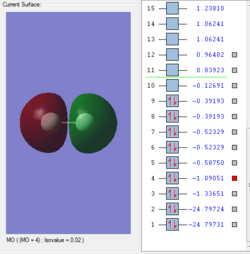
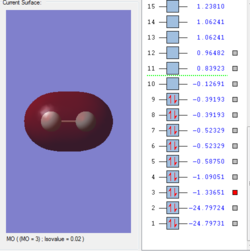
Molecular orbitals of F2
References
- ↑ Chemguide http://www.chemguide.co.uk/physical/equilibria/haber.html
- ↑ COBLENTZ SOCIETY Collection. http://webbook.nist.gov/cgi/cbook.cgi?ID=C7664417&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC