User:Hyl314
Introduction
The lowest energy species in a reaction profile is known as a minimum while the highest energy species is the transition state. Both species correspond to a turning point on the profile where gradient = 0 (dx/dy=0) with positive and negative gradients respectively. Gaussview was used to model the structure and energy of the reactants, transition states (cannot be obtained experimentally) and products of different reactions to predict the thermodynamic and kinetic products. Molecular orbital diagrams have also been drawn using information obtained from the calculations.
Exercise 1: Reaction of Butadiene with Ethylene
Bond Length
Diels- Alder reaction is a [4+2] cycloaddition that proceeds via a concerted mechanism through a cyclic transition state. As seen in the below reaction scheme, all the double bonds in the reactants lengthened from ~1.3 Å to ~1.5 Å in the product as the bond order decreased to one. The opposite is true for the single bond in butadiene which reduced in length from ~1.5 Å to ~1.3 Å as the double bond was formed. The intermediate bond lengths between single and double bonds in the transition state indicate partial single/ double bond character caused by bond breaking and forming in progress. All bond lengths in reactants and product show good agreement with literature (sp3-sp3 = 1.45 Å, sp3-sp2 = 1.50 Å, sp2-sp2 = 1.33 Å).[1] Bonding interaction between the terminal Cs of the reactants is confirmed as the bond length of the partially formed C-C bond is shorter than 2 x the Van de Waals radius (1.7 Å)[2] of C.

MO Diagram
The MO diagram of the reaction between Butadiene and Ethylene is shown in Figure1, note that only the HOMO and LUMO of the reactants are shown in the diagram.
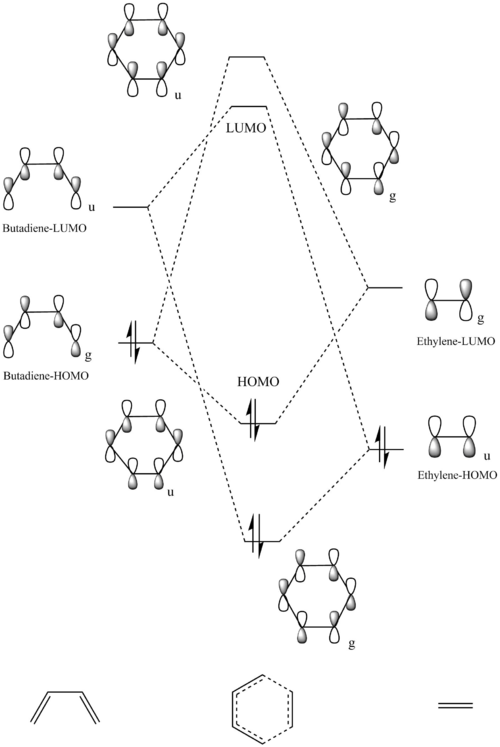
Symmetry of the orbitals are labelled as g (gerade, symmetric with respect to inversion) or u (ungerade, asymmetric with respect to inversion). Reactions will only happen between 2 orbitals if the overlap integral is non-zero which occurs when the orbitals have the same symmetry label. Therefore, only orbitals with the same symmetry label can react (e.g. g-g, u-u are allowed; u-g = forbidden) as illustrated in the MO diagram above.
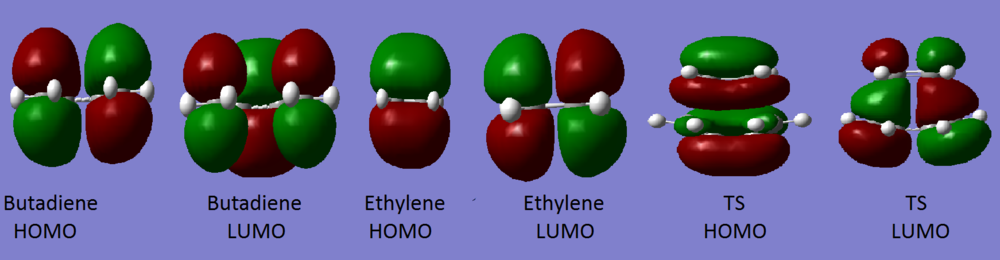
Nf710 (talk) 00:06, 13 January 2017 (UTC) U amd g suggest a cente of inversion which this doesnt have.
Vibrations
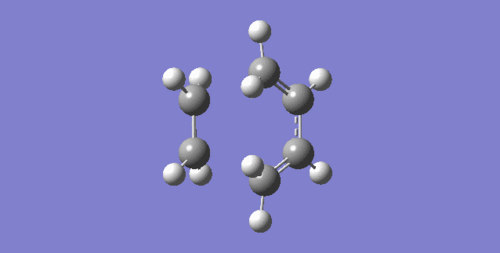
The imaginary frequency at -948.63 cm-1 corresponds to the reaction path at the transition state which shows a synchronous bond formation, which agrees with the concerted mechanism
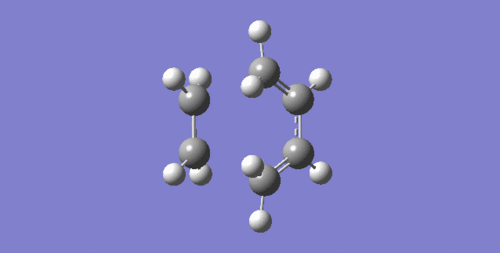
The lowest positive frequency at 144.94 cm-1 is asynchronous as shown below.
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
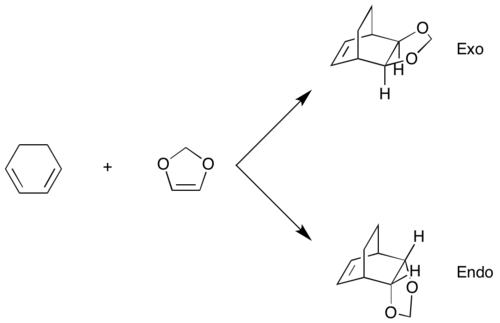
MOs
MOs of the 1,3-dioxole and cyclohexadiene are shown below, symmetry was labelled using the MO diagram in exercise 1 with 1,3-dioxole acting as the dienophile and cyclohexadiene as the diene.
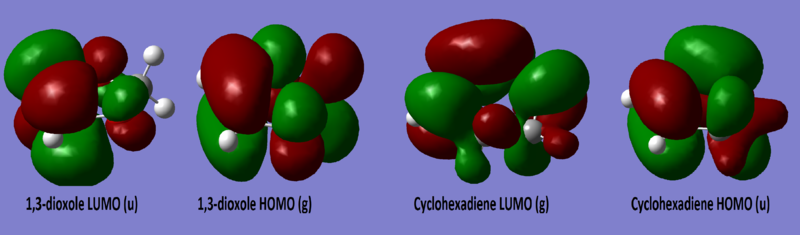
(These are the wrong symmetry labels. Gerade/ungerade symmetry require a centre of inversion Tam10 (talk) 13:28, 3 January 2017 (UTC))
Diels Alder reactions can either be normal electron demand (electron rich diene and electron poor dienophile) or inverse electron demand (electron poor diene and electron rich dienophile) depending on the nature of the reactants. The HOMO and LUMO of both endo and exo TS all show a gerade symmetry, therefore must be formed by the interaction of the 1,3-dioxole HOMO and cyclohexadiene LUMO as they are the only orbitals with the correct symmetry (g) to interact. This is due to the presence of electron rich O on 1,3-dioxole which raises the energy of its HOMO and LUMO, the overlap between the cyclohexadiene LUMO and the high energy 1,3-dioxole HOMO is now better than that of the cyclohexadiene HOMO and 1,3-dioxole LUMO, making this an inverse electron demand reaction.[4]
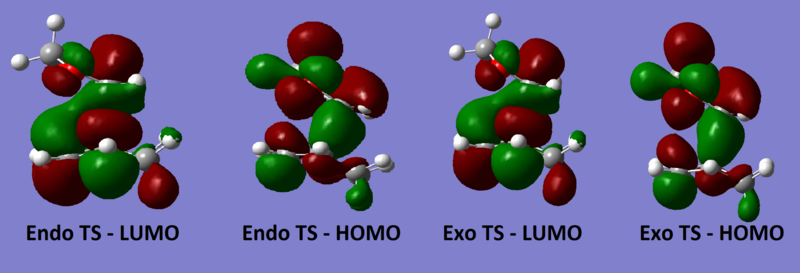
(Try to show the symmetry more explicitly here. It's hard to tell whether they are symmetric or asymmetric Tam10 (talk) 13:28, 3 January 2017 (UTC))
Reaction Energies and Stabilisation
Kinetic product of a reaction is the one that requires the lowest activation energy while the thermodynamic product is the most stabilised (lowest energy) conformer. In this reaction, the endo product has the lowest activation energy and is also more stabilised so it is both the kinetic and thermodynamic product and will be the only product formed in the reaction. The lower activation barrier is due to the secondary orbital interaction between the O lone pair in p orbital and the empty π* orbital at the back of the diene which lowers the energy of the endo transition state. This stabilisation is absent in the exo transition state as the π* orbital and the O lone pair are not in the correct orientation to interact, resulting in a higher activation energy.[5]
| Reactants | Transition State | Product | Activation Energy | ΔG | |
|---|---|---|---|---|---|
| Endo | -1313782 | -1313622 | -1313849 | 160 | -67 |
| Exo | -1313782 | -1313614 | -1313846 | 168 | -64 |

Nf710 (talk) 10:00, 23 January 2017 (UTC)Good section, excellent understanding of the electron demand of the system, the oxygens are not carbonyls. perhaps some jmols would have been nice.
Exercise 3: Diels- Alder vs Cheletropic
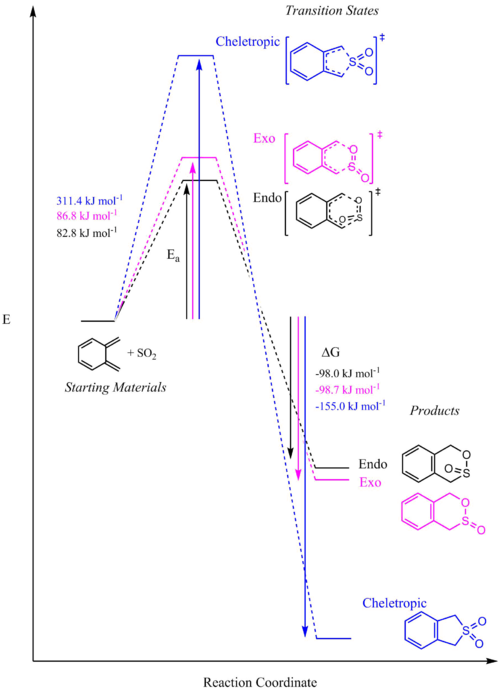
Xylylene and SO2 can react through a normal Diels-Alder reaction forming either the endo- or exo- product or through a competing cheletropic pathway as shown in the reaction scheme below. The activation energy and change in Gibbs free energy of each pathway was calculated to determine the most favourable reaction.
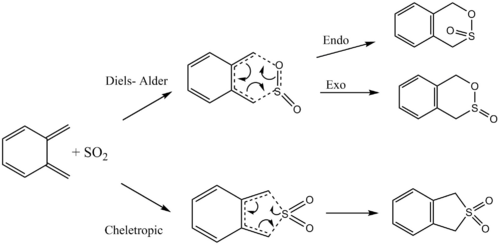
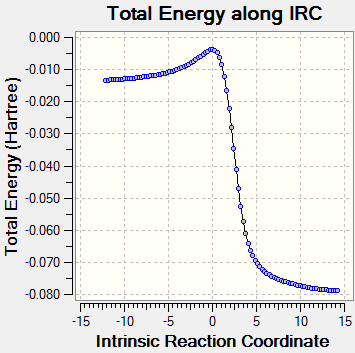
The starting material xylylene is not aromatic as it does not follow the Huckel rule (4n+2 π electrons) and the abundance of π bonds make it very reactive. During the reactions, the 6-membered ring is aromatised as shown in the below animations and is therefore a lot more stable, making the reactions thermodynamically favourable. (Note that the endo reaction pathway appears to be reverse here as Gaussian cannot identify product and reactants.
The starting material xylylene is not aromatic as it does not follow the Huckel rule (4n+2π electrons) and the abundance of π bonds make it very reactive. During the reactions, the 6-membered ring is aromatised as shown in the below animations and is therefore a lot more stable, making the reactions thermodynamically favourable. (Note that the endo reaction pathway appears to be reverse here as Gaussian cannot identify prduct and reactaIntrinsic Reaction Coordinate
| Endo Diels Alder | Exo Diels Alder | Cheletropic |
 |
 |
 |
 |
 |
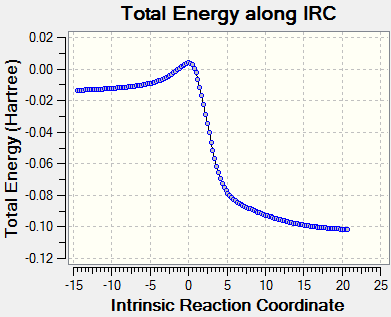 |
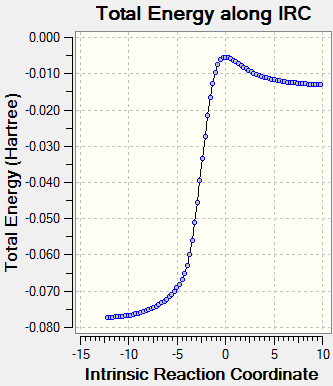
Reaction Profile
As seen in the reaction profile below, the endo Diels Alder pathway has the lowest activation energy, making the endo product kinetically favourable at low temperature (kinetic product). The exo Diels Alder product is more stabilised than its endo equivalent but has a higher activation barrier and therefore would not form if the reaction is under kinetic control (non-reversible). The cheletropic product is the most stabilised but also requires the highest activation energy to form, it is therefore the thermodynamic product.
Conclusion
Diels Alder reactions proceed via a concerted mechanism as demonstrated by the synchronous bond formation in exercise 1. Only orbitals of the same symmetry can interact, bonding interaction can be confirmed if the distance between two atoms is smaller than the sum of their Van de Waals radii. The reaction can form an endo or exo product depending on reaction condition, the endo product is kinetically more favourable as it has a lower activation barrier due to the secondary orbital interaction which stabilises the transition state. The reaction becomes reversible when enough energy is supplied to the system and will lead to the formation of the most stabilised thermodynamic product which can be either the endo or exo conformer.
- ↑ L. Pauling and L. Brockway, Journal of the American Chemical Society, 1937, 59, 1223-1236.
- ↑ S. Batsanov, Inorganic Materials, 2001, 37, 1031.
- ↑ Wiki.ch.ic.ac.uk, 2016.
- ↑ X. Jiang and R. Wang, Chemical Reviews, 2013, 113, 5515-5516.
- ↑ P. Alston, R. Ottenbrite and T. Cohen, The Journal of Organic Chemistry, 1978, 43, 1864-1867.
