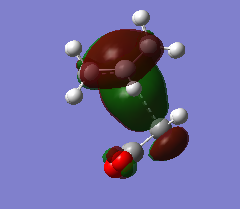User:Hc3613
Transition States and Reactivity of Pericyclic Reaction
Computational chemistry technique is more and more important, which concentrate geometric energies and quantum chemical dynamics.[1] In this experiment, transition structures of pericyclic reactions, specifically cope rearrangment and Diels-Alder cycloaddition, are studied by Gaussian software to investigate their thermoproperties as well as selectivity. Essentially, TS is determined by finding the saddle point on the potential energy surface(PES), which gives the energy of a molecule as a function of its geometry. To achieve this, first and second derivatives of energy are required to locate a stationary point where energy decrease in all direction, i.e. gradient = 0. Furthermore, geometry of reagents and products could be determined via minium energy pathway(MEP).In Gaussian software, molecular geometry is optimised by job 'opt', which aim to find the local minimal energy on PES. Vibration frequency is stimulated by job'freq' by calculating the force constant via second differentiation of energy.
Nf710 (talk) 10:46, 21 April 2016 (BST) Good intro could have gone into more detail with the methods
Cope Rearrangement
Optimizing the Reactants and Products
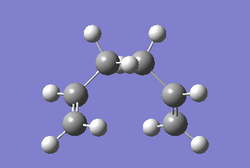
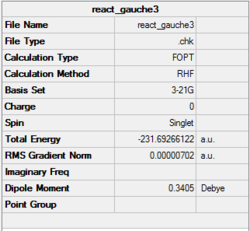
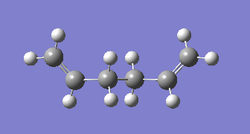
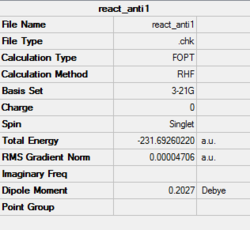
In this section, conformations of 1,5-hexadiene molecule were optimised using Gaussview at HF/3-21G level of theory. Structure of the the optimised conformer, point group, results summary and corresponded electronic energies are listed in Table 1. 1,5-hexadiene could be classified as Gauche and Anti conformer according to their structure, where Gauche1, Gauche3, Anti1 and Anti2 were optimised specifically in Gaussview. As Gauche conformation experience more steric hindrance compared to Anti conformation, Gauche conformation is predicted to be higher in electronic energy. However, results of optimisation at HF/3-21G level indicates that the electronic energy of Gauche 3 is lower than the Anti2, which is in contrast to the prediction. This may be resulted from the possible stereoelectronic interaction in Gauche 3 conformerbetween π* orbital of C=C bonds and sigma bonds of vinyl protons. If this interaction overweight the effect of steric hindrance, Gauche3 is lower in electronic energy. Optimisation at B3LYP/6-31G(d) level was applied on Anti2 conformer as well.Thermal energy and enthalpy terms of Anti 2 conformer of 1,5-hexidiene are summarized in Table 2. All the calculations are set to 298.15 K by default.
(1 hartree = 627.509 kcal/mol)
| Table 2.Energy and ethalpy terms of Anti 2 conformer of 1,5-hexadiene | |
|---|---|
| Sum of electronic and zero-point Energies | -234.416254 |
| Sum of electronic and thermal Energies | -234.408960 |
| Sum of electronic and thermal Enthalpies | -234.408016 |
| Sum of electronic and thermal Free Energies | -234.447872 |
Study of "Chair" and "Boat" Transition Structures
.

[3,3]-sigmatropic rearrangement, which is known as Cope rearrangement, migrates one sigma bond via concerted transition state shown in Figure 1. Cope rearrangment is reversible unless the product is more stable than reactant, which could be achieved by stablising group. In this section, two most possible transition structure, "chair" and "boat" transition structures are analysed and compared with different methods.
Chair Transition structure(C2h) was acheived by oriente two HF/3-21G optimised allylic fragments into anti-parallel position. The transition structure was then undergo optimisation+frequency at HF/3-21G level,with the transition structure is optimised to TS(Berny) with force constant calculation once. It is highly possible the calculation would fail if the predicted geometry of transition state is not close enough to the real geometry. Vibrations at imaginary frequency could be used to check whether the calculation is success. If just one vibration at imaginary frequency, and it's animation is exactly how the starting materials approch together, the optimisation is succuessful, otherwise an additional keyword "Opt=NoEigen" could be applied. However, sometimes "Opt=NoEigen" optimisation would lead the calculation to another wrong way. In this case, a freeze coordination method could be applied using additional keyword "Opt=ModRedundant" for optimisation to TS(Berny)at HG/3-21G level. This method freeze and relaxes the geometry while bond breaking/formation, and will be unfreezed at following process.
Both methods give similar and reliable results, animation of vibration at imaginary frequency shown in Fig.2. Separation between terminals is reduced to 2.02 Å(input separation is 2.2Å) and only one vibration at imaginary frequency -917.90cm-1 could be observed. Negative frequency is a result of negative force constant k. Force constant k is derived from equation ω=√(k/μ). Negative k value indicates a stationary point in nuclear configuration space.
.
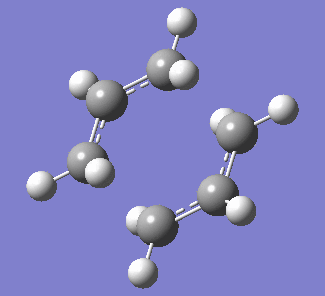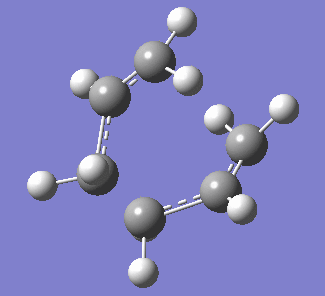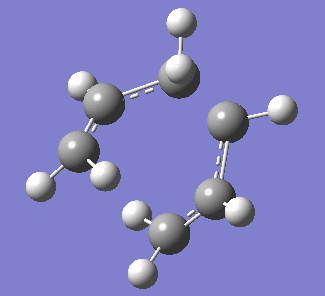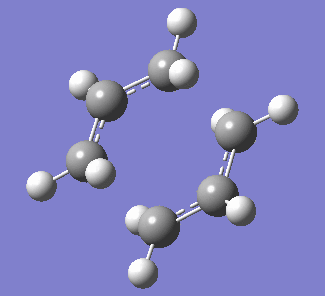
| Figure 3. Labelled reagent&product and vibration of optimised boat transition state at -839.96cm-1 | ||
|---|---|---|

|

| |
| Labelled reagent(Left) and Product(right) from anti 2 conformer | Vibration of boat transition state | |
Nf710 (talk) 10:49, 21 April 2016 (BST) Correct vibrations
Boat transition structure(C2v)was acheived via optimised anti2 1,5-hexadiene. The anti2 conformer of 1,5-hexadiene is used to construct the reactant and product, while the atom numbers are labeled specifically as shown in Figure 3. QST2 optimisation is then carried out. Since the rearrangment process cannot be regconized by Gaussian software, the structure of reactant and product need to be resembled into boat conformation in order to success. To resemble the reactant and product from anti2 conformer, the dihedral angel of C2-C3-C4-C5 need to be bented to 0o and the bond angle of C2-C3-C4 and C3-C4-C5 are set to 100o. The transtition structure is then predicted by finding the maximal potential energy when two molecules interact. HF/3-21G level using TS(QST2) method was applied, with force constant calculated once. Single imaginary frequency at -839.96cm-1 suggests that this optimisation is successful.
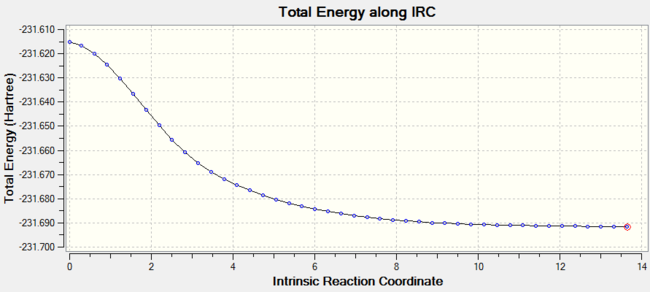
| Figure 4.Energy-coordination graph of chair TS |
|---|
|
To predict which conformer the reaction paths would follow, i.e. which transition structure will be formed, Intrinsic Reaction Coordination(IRC)of chair transition structure is applied. IRC method would follow the minimum energy path from the saddle point to the minimum potential energy surface by creating several points with small geometry steps. To get a reliable result, sufficiently large number of steps is required. In this experiment, 50, 100 and 150 geometry steps was applied. The IRC is suggested to be carried out at B3LYP/6-31G level. As the molecule is symmetry, the IRC is computing in forward direction only, and the force constant is always calculated. Energy coordination graph is shown in Figure 4.Interstingly, IRC result shows that the TS geometry resembles Gauche 2 rather than Gauche 3 although Gauche3 in lower in energy.
Nf710 (talk) 10:49, 21 April 2016 (BST) Correct conf. identified
| Table 3. Energy summary of chair and boat TS | ||||||
|---|---|---|---|---|---|---|
| HF/3-21G | B3LYP/6-31G(d) | |||||
| Compound | Electronic energy
(Hartree) |
Electronic energy
+ Zero-point energy at 0 K (Hartree) |
Electronic energy
+ Thermal energy at 298 K (Hartree) |
Electronic energy
(Hartree) |
Electronic energy
+ Zero-point energy at 0 K (Hartree) |
Electronic energy
+ Thermal energy at 298 K (Hartree) |
| Chair TS | -231.619318 | -231.466700 | -231.461341 | -234.571937 | -234.430455 | -234.404465 |
| Boat TS | -231.602798 | -231.450931 | -231.445302 | -234.558655 | -231.450931 | -231.399302 |
| Reactant from anti2 | -231.692370 | -231.539542 | -231.532565 | -234.611623 | -234.469218 | -234.461858 |
Electronic energy, electronic + zero-point energy (0 K), and electronic + thermal energy (298 K) of chair TS, boat TS and anti2 are calculated during optimisation at HF/3-21G and B3LYP/6-31G(d) level and are summarized in Table 3. Activation energy of cope rearrangement via chair or boat TS and summarized in Table 4. Calculation of activation energy are achieved by "Electronic energy + Thermal energy at 298 K" terms minius "Electronic energy" terms in table 3. It is found the minimised energy at B3LYP/6-31G(d) level is significantly lower than that of HF/3-21G level due to the larger basis set. Also, activation energy via chair TS is lower than boat TS from both level of calculation, which means chair TS is slightly more favoured compared with boat TS. The small energy difference might due to the small hydrogen group at terminal, which bear small steric hindrance on the boat TS when they are eclipsed. Experimental value of activation energy via chair TS is 33.5 ± 0.5kcal mol-1, boat TS is 44.7 ± 2.0kcal mol-1.
| Table 4.Activation energy of cope rearrangement via chair or boat TS at 298k(kcal mol-1) | ||
|---|---|---|
| calculation method | HF/3-21G | B3LYP/6-31G |
| Activation Energy via chair TS | 44.6937 | 36.0146 |
| Activation Energy via boat TS | 54.7583 | 39.2545 |
Nf710 (talk) 10:53, 21 April 2016 (BST) You have not got the correct energies unfortunately. but you have compared to experiment and come to the correct conclusions
Diels-Alder Cycloaddition
Diels-Alder reaction, which is also known as [4s+2s] concerted cycloaddition betwwen a diene with 4π electrons and a dienophile with 2π electrons. In Diels-Alder reaction, two π bonds are broken and two new σ bonds are formed. Normally electron demand with a electron-rich diene and electron-poor dienophile is favoured as HOMO of diene would be able to donate electrons to LUMO of dienophile. In this section, TS of two specific Diels-Alder reaction are computed and analysed. Optimisation all done at semi-empirical AM1 level.
Diels-Alder Cycloaddition between cis-butadiene and ethylene
.
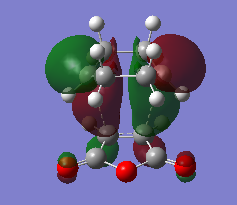
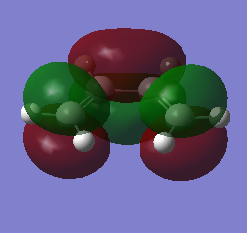
Cis-butadiene and ethylene were built and optimised via semi-emperical AM1 method. The highest occupied molecular orbital(HOMO) and lowest unoccupied molecular oribaltal(LUMO) are illustrated in Table 5. Syummetry of MO is determined with respect to the vertical mirror plane. As only orbitals with same symmetry would be able to interact, HOMO of butadiene (AS) with LUMO of ethylene (AS), LUMO of butadiene (S) with HOMO of ethylene (S) are two only possible interactions. However, due to the large energy difference between LUMO of butadiene and HOMO of ethylene, only the interaction between two AS orbitals in accessible.
| Table 5. HOMO and LUMO of reagents in prototypical cycloaddition | ||
|---|---|---|
| HOMO | LUMO | |
| Cis-butadiene | 
|
|
| Anti-symmetric(AS) | Symmetric(S) | |
| Ethylene | 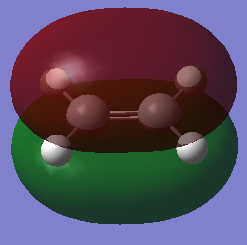
|
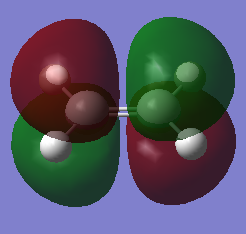
|
| Symmetric(S) | Anti-symmetric(AS) | |
The vibration modes of the protolypic TS are illustrated in Table 6. TS was computed using TS(Berny) method. Guess of geometry of TS was achieved via bicyclo-octant template. The -CH2CH2- fragment was replaced by two hydrogen atoms. Typical bond length between sp3 C-C single bons are set to 1.54Å, sp2 c=c bons are set to 1.33Å and the interfragment distance are set to 2.2Å), which is the supposed distance between TS carbon atoms. Optimisation of the TS was then carried out using TS(Berny) method. If the guess geometry is not close enough to the actual geometry of TS, error might happen during the calculation. To solve this, additional keyword "Opt=noeigen" is used to allow the calculation to continue. As only one imaginary vibration is observed, the calculation is successed and the calculated TS is good.
| Table 6. Vibration of Transition strucure of prototypical cycloaddition | |
|---|---|

|

|
| Vibration at -956.23cm-1 | Vibration at lowest positive frequency 147.19cm-1 |
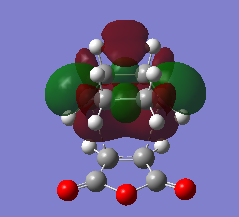
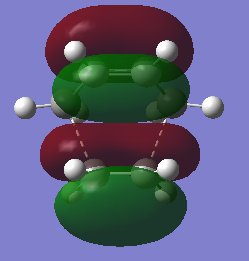
HOMO and LUMO of TS of prototypical cycloaddition are shown in Table 7 together with their relative energy. Interestingly, energy difference between HOMO and HOMO-1 (approximately 0.002) is much more smaller then difference between HOMO and LUMO(approximately 0.01), which means they might crossover in some cases.
| Table 7. Frontier orbitals of transition state of prototypical cycloaddition | |||
|---|---|---|---|
| Orbitals | 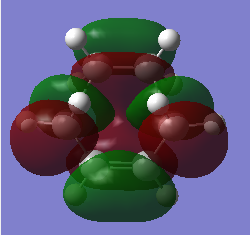
|
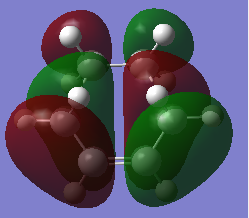
|
|
| LUMO | HOMO | HOMO-1 | |
| Symmetry | S | S | AS |
| Relative energy | 0.14242 | -0.30073 | -0.30286 |
(While what's here is good, this section is incomplete. You need to talk about the intramolecular distances in the TS comparing them to Van der Waals lengths, and whether the reaction is synchronous or asynchronous. Tam10 (talk) 17:41, 7 April 2016 (BST))
Regioselectivity of Diels-Alder reaction
.

Diels-Alder reaction in regioselective when different fuctional groups attached to the terminals of diene and dienophile. In this section, regioselectivity of Diels-Alder reaction between 1,3-cyclohexadiene and maleic anhydride is studied. This reaction could be either endo or exo selective due to the presence of secondary orbital overlap. Reagents, endo and exo TS are minised using semi-emperical AM1 method, while both TS are computed by QST2. Frontier orbitals of reagents are illustrated in Table 8. Furthermore, endo-selectivity displays inverse electron demand, where LUMO of diene interacts with HOMO of dienophile.[2]
In this reaction, secondary orbital overlap is happened at Endo TS as shown in Figure 6, where pz orbital from C=O interacts with C=C pz. If this stereoelctronic interaction outweight the steric repulsion, endo product would be formed via endo TS. Otherwise, if steric hindrace dominates, exo product would be formed, as C=O bond clashes with the C-H bond on hexadiene on exo TS.
(You're looking in the right place, but your phases are incorrect for maleic anhydride. Also, your last sentence seems slightly off Tam10 (talk) 17:41, 7 April 2016 (BST))
| Table 8. Frontier Oribitals of reagents of regioselective Diels-Alder cycloaddition | ||
|---|---|---|
| Orbital | HOMO | LUMO |
| 1,3-cyclohexadiene | 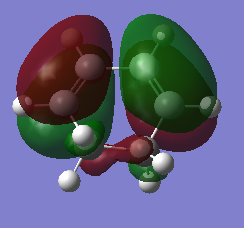
|

|
| AS | S | |
| maleic anhydride | 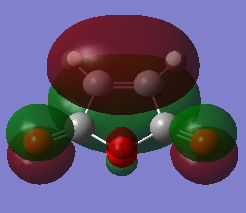
|

|
| S | AS | |
Distance between atoms at Exo and endo TS are shown in Table 10. In both endo and exo TS, two σ-bonds are formed via a 6 membered ring, thus bond length should be symmetric. In endo TS, the partially formed σ-bonds are shorter due to the presence of stereoelectronic interaction. In exo TS,these bonds are longer due to the steric hindrace.
Frontier orbitals of two TS are illustrated in Table 9. Stereoelectronic interaction could be observed in the HOMO of endo TS.
(Your endo and exo TS structures are the same. You have two extra hydrogens on each structure Tam10 (talk) 17:41, 7 April 2016 (BST))
Summary of reaction energy via exo and endo TS are summarized in Table 11. Energies calculated during optimisation at semi-empirical AM1 level, with TS optimised in QST2 job. Reaction between 1,3-cyclohexadiene and maleic anhydride reach a much deeper energy in compare with that of Cope rearrangement, as two weak π bonds are broken and two much stronger σ bonds are formed. As shown in Table 11, activation energy of endo TS is lower than that of exo TS, which states endo pathway is kinetically more favourable. Energy of exo product is lower than that of endo product, which indicates the exo pathway is thermodynamically more favourable.
| Table 11. Summary of reaction energy via exo and endo TS by AM1 calculation | |
|---|---|
| E1,3-cyclohexadiene + Emaleic anhydride (Hartree) | -605.646702 |
| Eendo TS (Hartree) | -605.61485 |
| Eendo product | -605.721025 |
| Eactivation via endo TS (Hartree) | 0.031852 |
| Eexo TS (Hartree) | -605.604968 |
| Eactivation via exo TS (Hartree) | 0.041734 |
| Eexo product | -605.717852 |
(These look like Hartree-Fock energies. Where are the files for these? Tam10 (talk) 17:41, 7 April 2016 (BST))
Further Discussion
Side reaction during Diels-Alder reaction might be happened, which leads to undesired product. For example, [2+2] cycloaddition between two alkene, or [4s+2s] dimierasation between diene and the product alkene. The transition state and activation energy of these pathways could be calculated to see its thermodynamic and kinetical properties.
Conclusion
This study mainly based on Gaussian software. Optimisation of molecules are always calculated at HF/3-21G, B3LYP/6-31G(d) and semi-emperical AM1 levels of theory. Vibration frequency, TS(Berny) and TS(QST2) methods are used when constructing transition state of reactios. IRC is also studied for simulating chemical path.
In Cope rearrangementm chair TS is favoured over boat TS as less steric repulsion is applied. Diels-Alder cycloaddition between butadiene and ehtylene is also well studied,which is [4s+2s] concerted with normal electron demand.. Regioselectivity of Diels Alder reaction is investigated by reaction between 1,5-hexadiene and melaic anhydride. Endo pathway is kinetically more favourable due to the presence of secondary orbital interaction. Exo pathway is thermodynamically more favourable as a more stable product is formed.
Reference
[1]Gaussian 03 Revision, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., etc, Gaussian, Inc., Wallingford CT, 2004.
[2]Ian Fleming, Molecular Orbitals and Organic Chemical Reactions, 2010, 6, 295-316